INTRODUCTION
A pyogenic liver abscess (PLA) is a serious clinical entity with a high morbidity and mortality rate. The most common cause of PLA is biliary disease. However, some PLAs do not have an obvious underlying cause, that is, are cryptogenic, although colonic lesions, such as diverticulitis, have been identified as a plausible infectious route for portal-flow bacteremia or intraperitoneal bacterial spreading to the liver.
1 Liver cyst infection can also occur in patients with autosomal dominant polycystic kidney disease, even though a simple liver cyst infection in those without underlying disease is extremely rare.
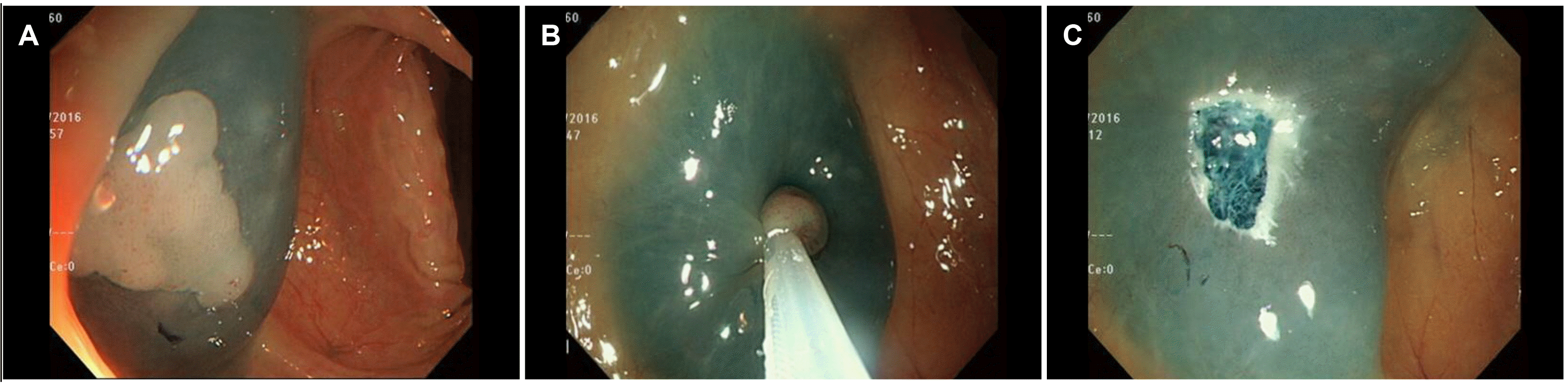
Colonoscopy is a gold standard for the diagnosis and treatment of colorectal pathology. On the other hand, although rare, there are serious infectious complications following colonoscopy, particularly when polypectomy is performed. Some studies have reported PLA complicated after a colonoscopic polypectomy.
2,3 This paper reports three unusual cases of PLA or liver cyst infection occurring after colonoscopic polypectomy. These cases suggest that colonic origin might be an important source of PLA (
Table 1).
Table 1
Baseline and Clinical Characteristics of the Three Patients
|
Case |
Sex/age |
Risk factorsa
|
Clinical |
symptoms |
Time from colonoscopy to PLA |
Location (L/R) |
Microbiology |
Treatment |
|
Case 1 |
M/58 |
No |
Fever, chill, altered |
mentality |
1 week |
R |
Klebsiella pneumoniae
|
Antibiotics, sono-guided aspiration |
|
Case 2 |
F/58 |
No |
Fever, chill, left subcostal |
pain |
6 days |
L |
No growth |
Antibiotics, sono-guided aspiration |
|
Case 3 |
M/84 |
No |
Fever, general |
weakness |
2 weeks |
R |
No growth |
Antibiotics, percutaneous drainage |

Go to :

DISCUSSION
The incidence of PLA ranges from 10 to 20 cases per 100,000 hospital admissions.
4 The prognosis of PLA has improved with the advances in diagnostic and therapeutic techniques. Nevertheless, early diagnosis of PLA is essential because its mortality rate remains at approximately 10%.
5 On the other hand, its diagnosis can be delayed because of its largely nonspecific presentation unless the clinician maintains a high level of suspicion. A retrospective study reported that only 59%, 39%, and 14% of PLA patients present with fever, right upper quadrant pain, and peritoneal signs, respectively. Therefore, its diagnosis is often delayed for an average of 1 week from symptom onset.
6
The routes of invasion include the biliary tree, portal and systemic circulation, direct intraperitoneal extension, and penetrating trauma. In the past, the most common cause of PLA was appendicitis complicated by pylephlebitis. With the introduction of effective antibiotic therapy, this has become a rare cause of PLA. The most common cause of PLA is biliary tract disease, such as cholangitis.
7 Nevertheless, approximately 35% of PLA cases remain cryptogenic.
6 Some cryptogenic PLA cases are probably associated with colonic mucosal lesions caused by a range of conditions, such as colon neoplasm,
7-9 inflammatory bowel disease,
10 and intestinal tuberbulosis.
11 In addition, there are some reports of PLAs related to colonic procedures,
2,3,12-14 in which most cases have underlying disease disrupting colon mucosa, such as ulcerative colitis,
12 tubulovillous adenoma,
13 and adenocarcinoma.
2,3 One case occurred 1 week after a screening colonoscopy without intervention. This case, however, had sigmoid diverticulosis, which might be a route for bacteremia after a colonoscopic examination.
14 For cases of PLA following colonoscopic polypectomy, advanced adenoma (tubulovillous adenoma)
13 and adenocarcinoma
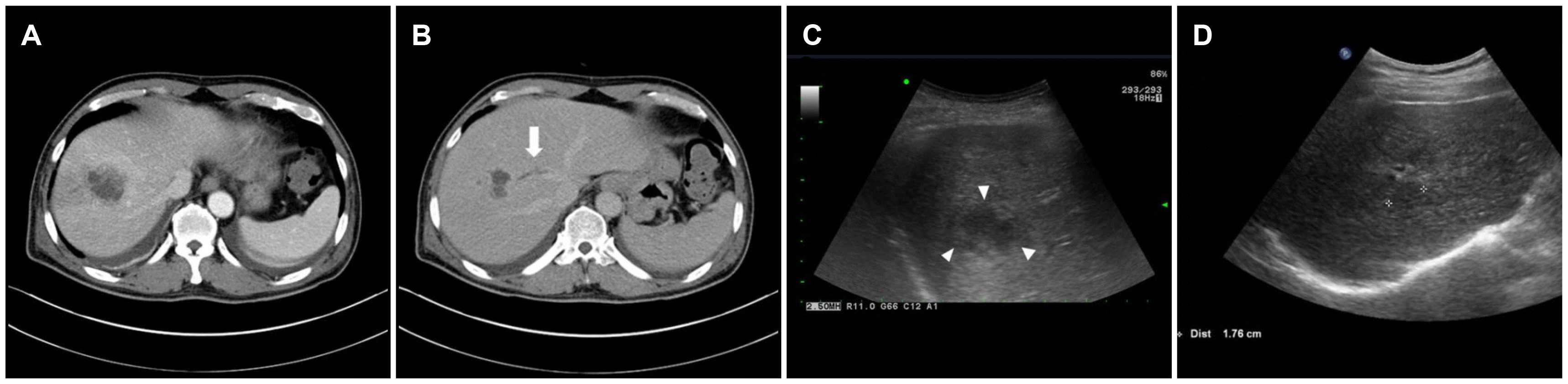
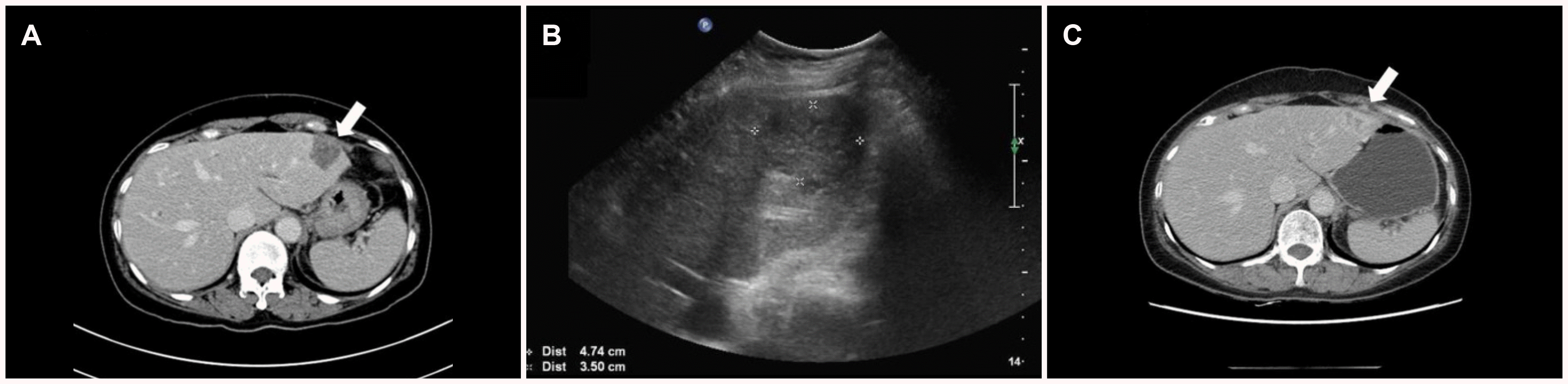
2,3 can cause a mucosal defect that provides a route for the bacteria to invade the portal system or spread intraperitoneally to the liver. Therefore, PLA in these cases might have originated from an underlying colon disease rather than from a colonoscopy. In contrast, in the present cases, there was no evidence of colon neoplasm or inflammation that could disrupt the colon mucosa. Two patients, cases 1 and 3, had only small (<1 cm) tubular adenoma. The other case (case 2) denied a colon malignancy for her endoscopic pathology (no available data). Therefore, their PLA or liver cyst infection might have been due to mucosal disruption occurring after colonoscopic polypectomy.
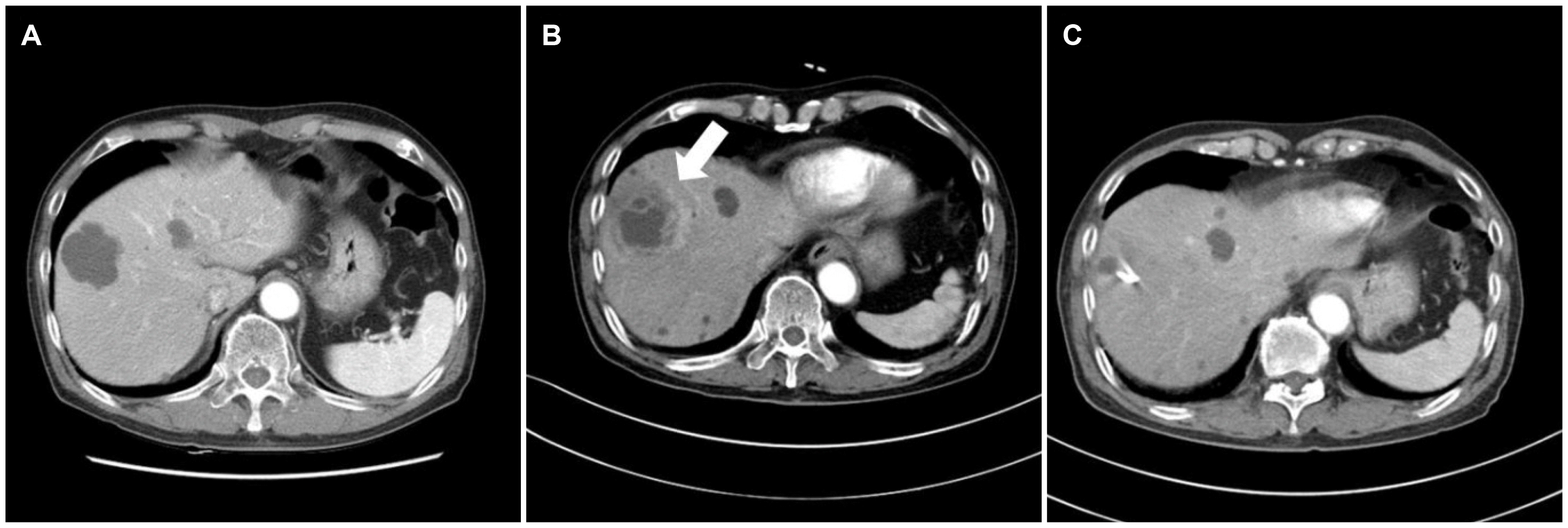
A liver cyst infection is rare, occurring mostly in patients with autosomal dominant polycystic kidney disease.
15 Only one report of a liver cyst infection after colon endoscopic mucosal resection in a patient with autosomal dominant polycystic kidney disease could be found.
16 The present patient had no predisposing factors except for old age. To the best of the authors’ knowledge, this is the first case of a simple liver cyst infection after a colonoscopic polypectomy.
Klebsiella pneumoniae is the most common pathogen isolated from PLA. Several studies have suggested that
Klebsiella pneumoniae is associated with PLA of a colonic origin.
7,17 In case 1,
Klebsiella pneumoniae was identified in the blood culture, suggesting the possible hematogenous spread of bacteria via the mucosal defect caused by the colonoscopic polypectomy. In cases 2 and 3, the negative results in blood and pus culture could be due to the following two reasons: 1) the pathogen might be an anaerobe that was a colonizer in the colon; 2) the patient had been partially treated at an outside hospital.
In the present cases, the risk factors of PLAs, such as diabetes mellitus, previous biliary operation, or immune suppression, could not be found. In the literature, the rate of bacteremia related to colonoscopy was 0-25%. This was not associated with infectious complications.
18 One hypothesis was that inadequate bowel preparation might influence bacteremia from the colon. However, no study has dealt with this topic yet. In this context, more studies will be needed to determine the risk factors for PLA after a colonoscopy.
In conclusion, the present cases suggest that colonic mucosal lesions caused by a colonoscopic polypectomy should be considered possible causes of cryptogenic PLA. Clinicians should be aware of the possibility of PLA if a patient complains of fever and abdominal pain after a colonoscopic polypectomy.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download