Abstract
This paper reports a case of a twisted small bowel obstruction in a 74-year-old man that occurred after a double tract reconstruction (DTR) in a laparoscopic proximal gastrectomy (PG) for early gastric cancer. The patient had inadequate oral intake and reflux symptoms for 10 days after discharge. Imaging analysis revealed a narrowed small bowel with twists between the esophagojejunostomy and gastrojejunostomy sites. A fully covered stent was placed in the narrowed small bowel for 2 weeks. The patient was then discharged after stent removal without any dietary problems. The authors’ experience shows that twisted small bowel after a DTR in PG can be treated by endoscopy.
Go to : 
Proximal gastrectomy (PG), a function-preserving form of gastrectomy, is a promising option for patients with upper third early gastric cancer.1 Although a total gastrectomy is the main surgical procedure for upper third gastric cancer, PG is an attractive option for cases of early gastric cancer because of the nutritional benefits associated with this technique.2,3 A double tract reconstruction (DTR) is a typical method for PG anastomosis4 and represents a form of anastomosis that prevents reflux by maintaining the distance between the gastric remnant and the esophagus. On the other hand, rotation of the small bowel can occur between the esophagojejunostomy and gastrojejunostomy sites due to adhesion or rotation of the remnant stomach. This paper presents a case of a 74-year-old man who experienced obstruction symptoms after laparoscopic PG with DTR, which was managed endoscopically.
Go to : 
A 74-year-old man was diagnosed with early gastric cancer. The endoscopic findings revealed a type IIc early gastric cancer lesion, approximately 15 mm in size, at the posterior wall of the cardia to the fundus. Initially, the patient underwent endoscopic submucosal dissection for the treatment of early gastric cancer. Subsequent pathological analysis showed that the primary tumor had invaded the submucosa by 1.4 mm. The patient was transferred to the gastric cancer surgery department and underwent laparoscopic PG. The patient started taking sips of water on day 3 postoperatively, and was discharged 6 days after surgery without complications.
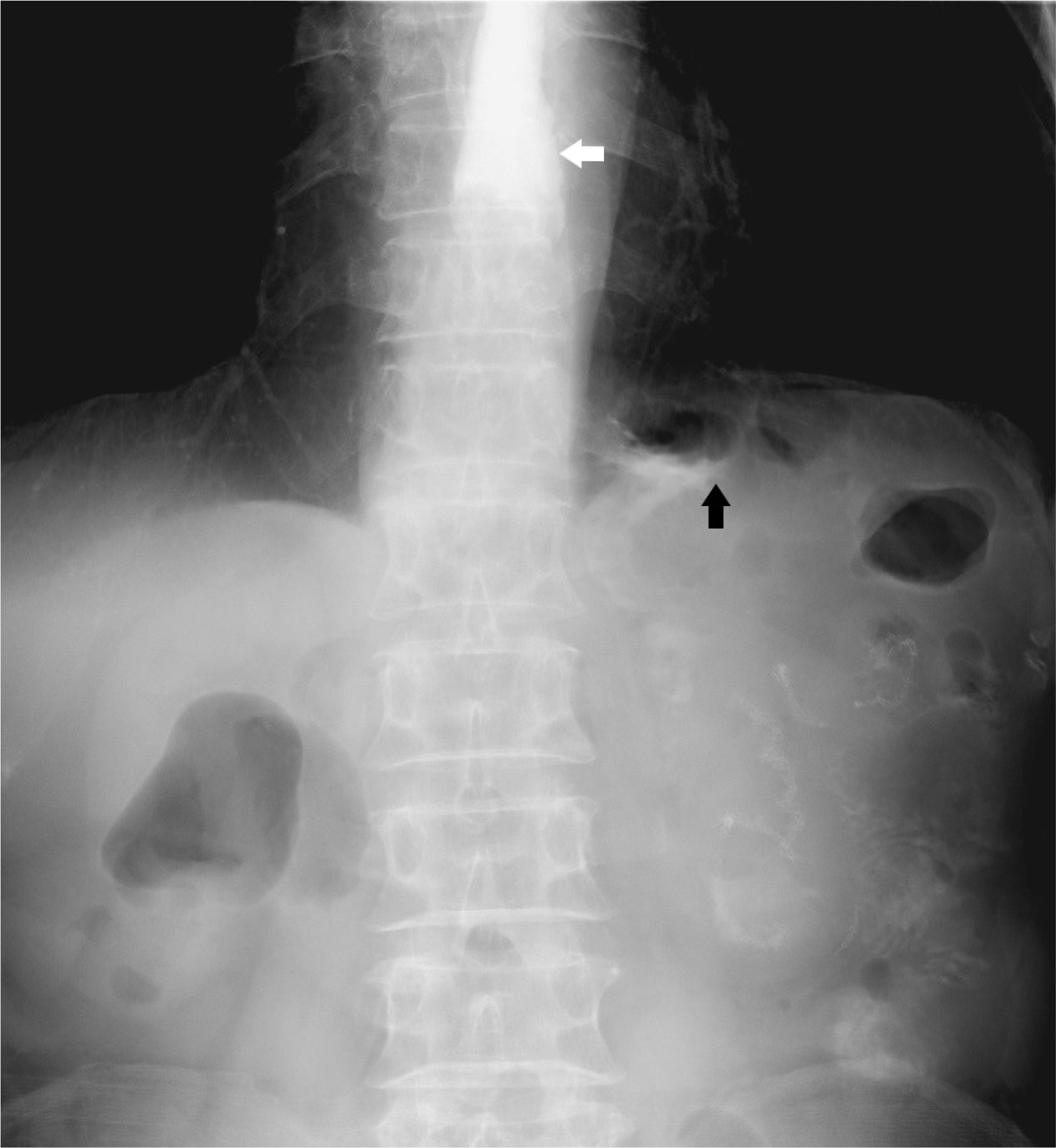
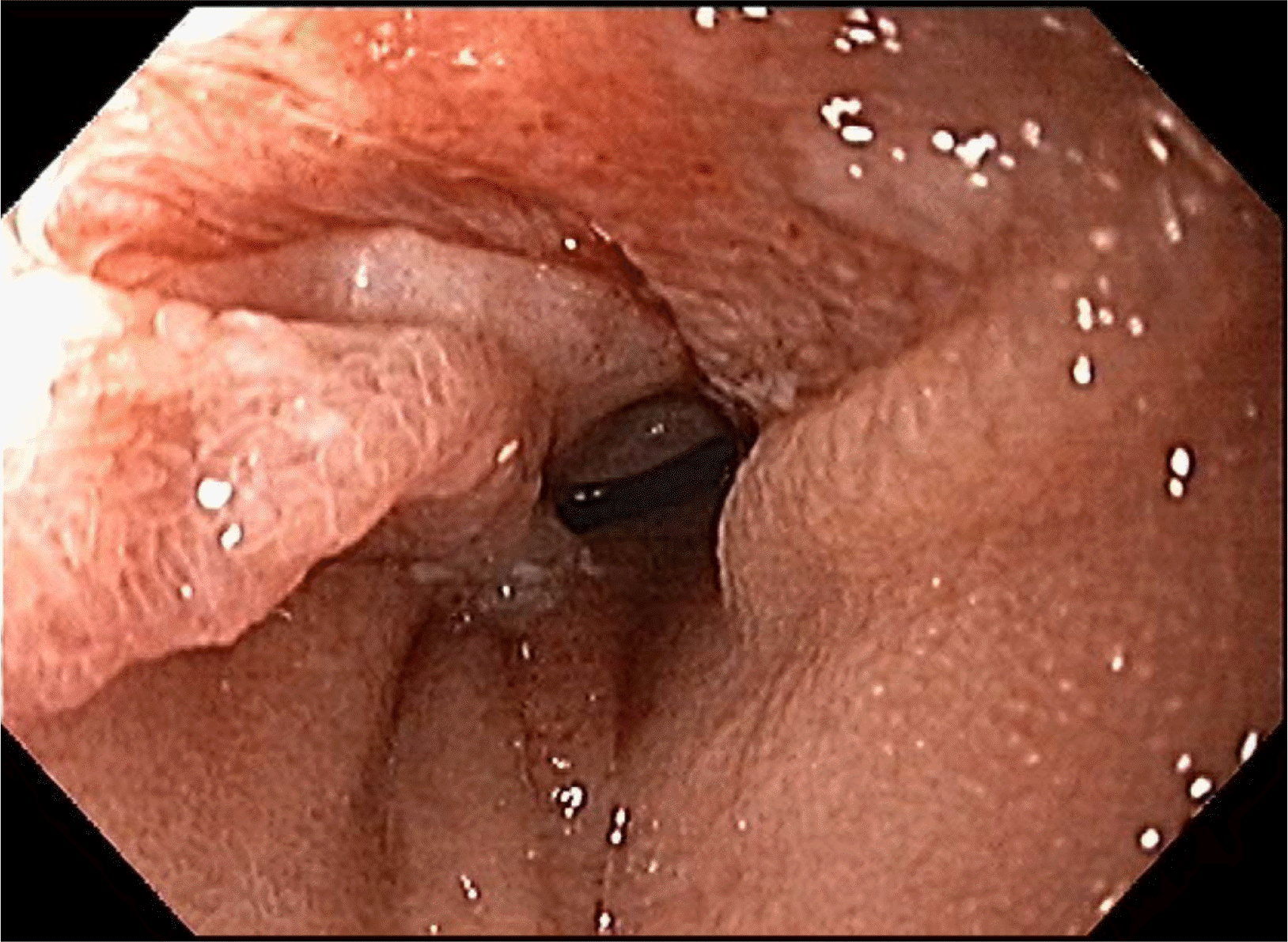
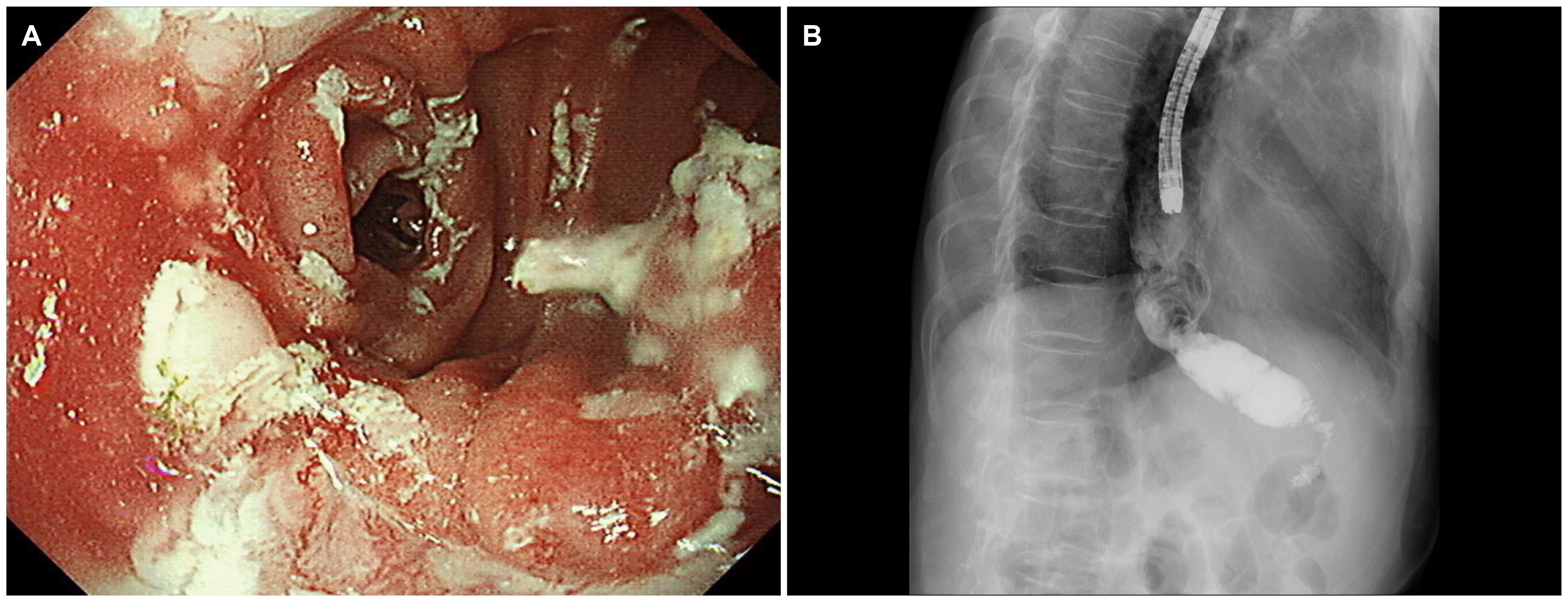
Ten days after discharge, the patient visited an outpatient department owing to inadequate oral intake and reflux symptoms. On examination, the patient’s vital signs were within normal limits. The abdomen was soft but distended slightly in the upper part. An upper gastrointestinal series revealed a narrowing lumen between the esophagojejunostomy and gastrojejunostomy sites and a dilated esophagus (Fig. 1). The endoscopic findings showed that the lumen of the small bowel was twisted and narrowed (Fig. 2). The endoscope could be passed through the narrow lumen of the small bowel without resistance. Therefore, the patient was followed up with further procedures and was allowed to drink water after the examination.
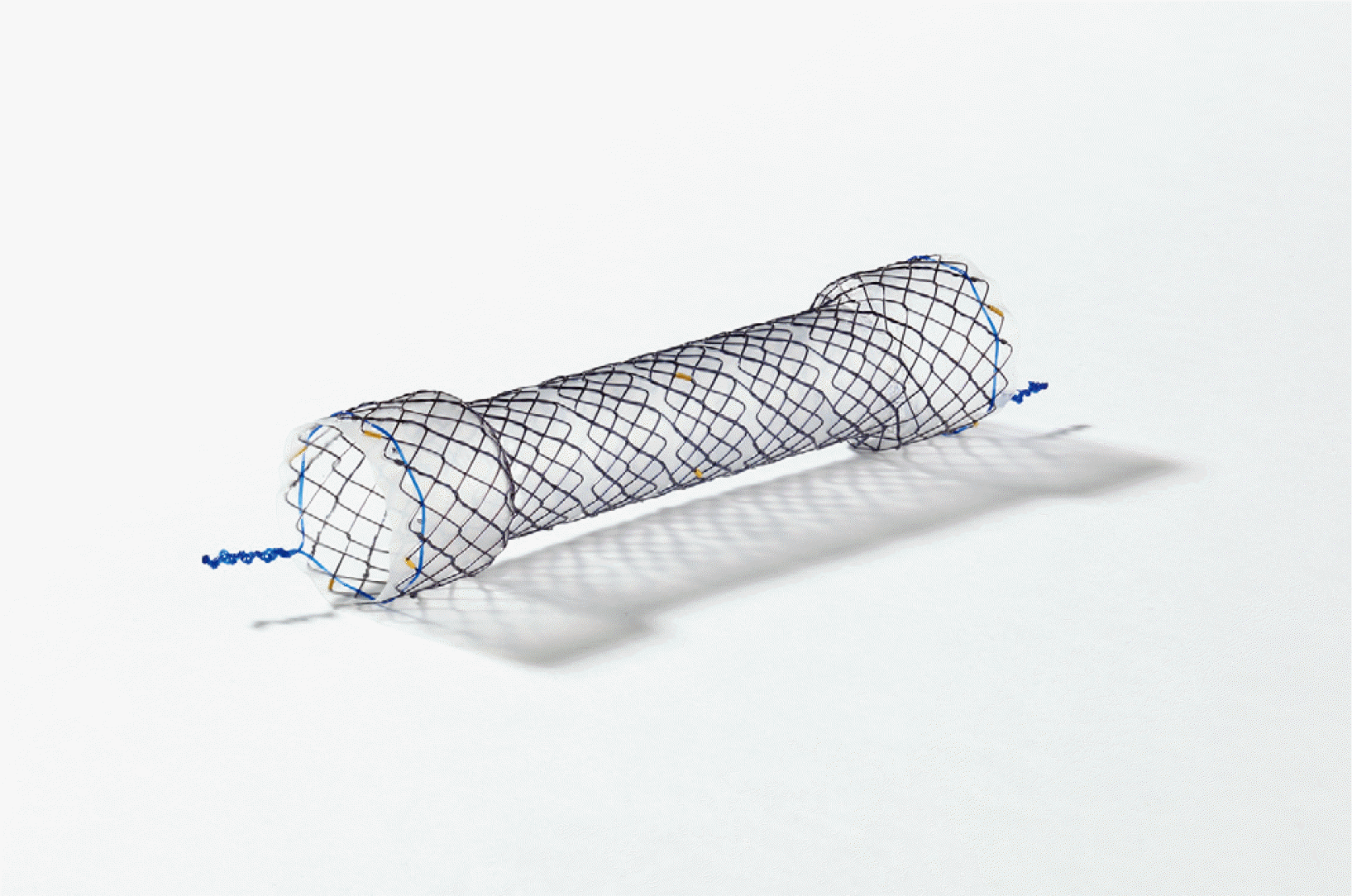
The patient could consume a liquid diet but complained of vomiting and intake difficulties when the diet was changed to include more solid foods. The patient underwent an endoscopic procedure with a covered stent, 80 mm in length (HANAROSTENT®; M.I.Tech, Seoul, Korea) (Fig. 3), to correct the narrowing of the small bowel. The central part of the fully covered stent was placed 3 cm below the esophagojejunostomy site, which was noted as the narrowest position (Fig. 4). The patient’s diet was changed from a liquid diet to a soft diet, which was tolerable.
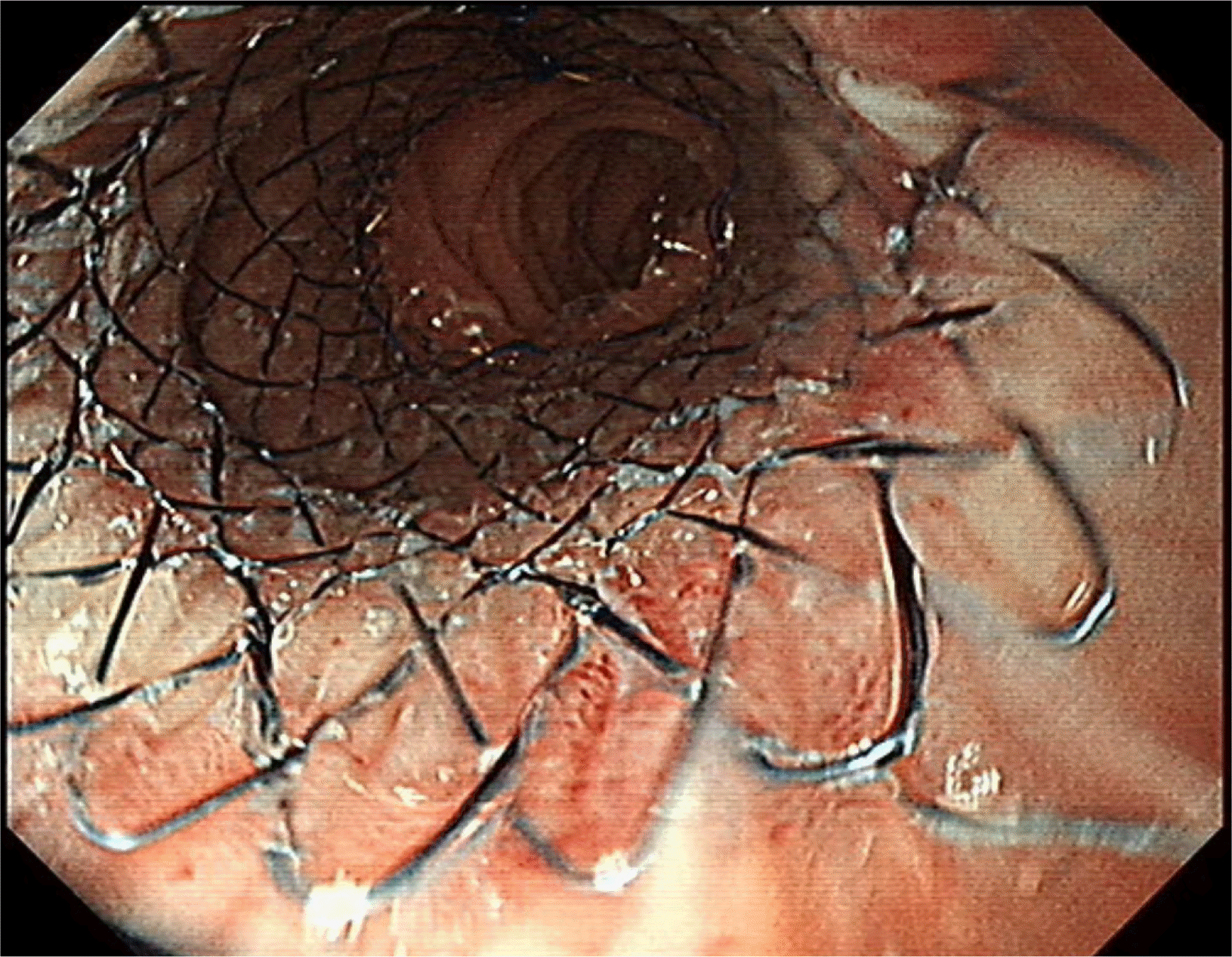
Two weeks after stent replacement, X-ray analysis (simple abdomen) showed that the stent had migrated to the distal part of the small bowel. The stent was removed endoscopically, and the narrowed small bowel lumen was dilated sufficiently via the passage of contrast media (Fig. 5). The patient was discharged 3 days after stent removal without dietary problems.
Go to : 
Postoperative adhesion is an inevitable complication of surgery. Most non-surgical treatments can resolve small bowel obstructions or ileus caused by adhesion. On the other hand, surgical treatment may be required in some cases.5 DTR is the most common form of anastomosis after PG and consists of three separate procedures: esophagojejunostomy, gastrojejunostomy, and jejunojejunostomy. This procedure was performed at least 10-15 cm between the esophagojejunostomy and gastrojejunostomy sites to prevent reflux. Obstructive symptoms may ensue if the small bowel between the sites of esophagojejunostomy and gastrojejunostomy becomes twisted by adhesion or by rotation of the remnant stomach, as in the present case. A narrowed small bowel is more likely to be obstructed partially than be obstructed completely. Therefore, an endoscope can be passed through with minimal pushing force, and the patient should be able to consume a liquid diet after that. On the other hand, these symptoms require surgery, or other interventions, as nutritional deficiency may occur because the patient cannot consume a normal diet. Endoscopic treatment was used primarily for the present case described herein because the narrowed small bowel became wider after the insertion of a fully covered stent for approximately 2 weeks.
Several previous reports have provided evidence-based recommendations for the upper gastrointestinal tract stenting for both benign and malignant conditions.6,7 The present case involved the occurrence of a benign stricture after surgery. Previous studies have shown that such cases are a possible indication for stent insertion.8 Endoscopy should be considered for cases involving small bowel adhesion and obstruction for two reasons: cases of partial small bowel obstruction rather than complete small bowel obstruction, and because twists caused by adhesion or rotation of the stomach may resolve over time. In the present case, stent insertion was the first treatment option during the resolution period. Surgical treatment may be considered if symptoms persist after the procedure, and endoscopic findings show a narrowing of the small bowel.
The stent can migrate if the stent insertion is performed in the small intestine. Such migration needs to be addressed because it can cause serious complications, including intestinal perforation.9,10 In the present case, there were no obstructive symptoms following stent migration, and the diet was well accepted by the patient after stent removal. After 2 weeks of stent placement, the movement of the small bowel returned. Therefore, peristaltic movements of the small bowel may have contributed to stent migration. Consequently, it is important to monitor the location of the stent during the follow-up period.
Covered stent insertion was selected as a primary treatment, and the stent was removed after its migration. On the other hand, small bowel obstruction or perforation can occur by inserting a fully covered stent in the case of small bowel stenosis with a minor degree.11 In this case, balloon dilatation rather than fully covered stent insertion should be considered as an initial treatment. If the initial treatment requires a consideration of fully covered stent insertion, stents with Shim’s technique, clip fixation, specially designed anti-migratory stents, and partially covered stents can be considered for the prevention of stent migration.12,13
The present case is atypical because PG surgery is still performed only in cases of early gastric cancer and because there is a range of anastomosis methods in addition to DTR. As the prevalence of early gastric cancer increases, however, the number of surgeries involving PG (function-preserving gastrectomy) is expected to increase. Endoscopic treatment, rather than surgical treatment, may be considered for small bowel narrowing due to twisted small bowel obstruction, which is one of the complications after DTR. In conclusion, twisted small intestinal obstruction after DTR in PG can be treated using an endoscopic stent.
Go to : 
REFERENCES
1. Japanese Gastric Cancer Association. 2017; Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 20:1–19. DOI: 10.1007/s10120-016-0622-4. PMID: 27342689. PMCID: PMC5215069.
2. Jung DH, Lee Y, Kim DW, et al. 2017; Laparoscopic proximal gastrectomy with double tract reconstruction is superior to laparoscopic total gastrectomy for proximal early gastric cancer. Surg Endosc. 31:3961–3969. DOI: 10.1007/s00464-017-5429-9. PMID: 28342130.

3. Park JY, Park KB, Kwon OK, Yu W. 2018; Comparison of laparoscopic proximal gastrectomy with double-tract reconstruction and laparoscopic total gastrectomy in terms of nutritional status or quality of life in early gastric cancer patients. Eur J Surg Oncol. 44:1963–1970. DOI: 10.1016/j.ejso.2018.08.014. PMID: 30197164.

4. Information Committee of Korean Gastric Cancer Association. 2016; Korean Gastric Cancer Association nationwide survey on gastric cancer in 2014. J Gastric Cancer. 16:131–140. DOI: 10.5230/jgc.2016.16.3.131. PMID: 27752390. PMCID: PMC5065942.
5. Moris D, Chakedis J, Rahnemai-Azar AA, et al. 2017; Postoperative abdominal adhesions: clinical significance and advances in prevention and management. J Gastrointest Surg. 21:1713–1722. DOI: 10.1007/s11605-017-3488-9. PMID: 28685387.

6. Jee SR, Cho JY, Kim KH, Kim SG, Cho JH. Stent Study Group of the Korean Society of Gastrointestinal Endoscopy. 2013; Evidence-based recommendations on upper gastrointestinal tract stenting: a report from the stent study group of the korean society of gastrointestinal endoscopy. Clin Endosc. 46:342–354. DOI: 10.5946/ce.2013.46.4.342. PMID: 23964331. PMCID: PMC3746139.

7. Kang HW, Kim SG. 2015; Upper gastrointestinal stent insertion in malignant and benign disorders. Clin Endosc. 48:187–193. DOI: 10.5946/ce.2015.48.3.187. PMID: 26064817. PMCID: PMC4461661.

8. Kim SG, Yang CH. 2012; Upper gastrointestinal stent. Clin Endosc. 45:386–391. DOI: 10.5946/ce.2012.45.4.386. PMID: 23251886. PMCID: PMC3521940.

9. Madruga Neto AC, Brunaldi VO, Okazaki O, et al. 2018; Stent migration requiring surgical removal: a serious adverse event after bariatric megastent placement. Endoscopy. 50:E344–E345. DOI: 10.1055/a-0725-7718. PMID: 30332690.

10. Karagul S, Yagci MA, Ara C, et al. 2015; Small bowel perforation due to a migrated esophageal stent: report of a rare case and review of the literature. Int J Surg Case Rep. 11:113–116. DOI: 10.1016/j.ijscr.2015.04.030. PMID: 25967554. PMCID: PMC4446686.

11. Bae JS, Kim SH, Shin CI, et al. 2015; Efficacy of gastric balloon dilatation and/or retrievable stent insertion for pyloric spasms after pylorus-preserving gastrectomy: retrospective analysis. PLoS One. 10:e0144470. DOI: 10.1371/journal.pone.0144470. PMID: 26657405. PMCID: PMC4675538.

12. Shim CS, Cho YD, Moon JH, et al. 2001; Fixation of a modified covered esophageal stent: its clinical usefulness for preventing stent migration. Endoscopy. 33:843–848. DOI: 10.1055/s-2001-17326. PMID: 11571679.

13. Martins BC, Retes FA, Medrado BF, et al. 2014; Endoscopic management and prevention of migrated esophageal stents. World J Gastrointest Endosc. 6:49–54. DOI: 10.4253/wjge.v6.i2.49. PMID: 24567792. PMCID: PMC3930890.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download