CASE: A 40-year-old woman presented with complaints of intractable ulcers over her legs that had been present for 2 years. An ulcer on her left leg began 2 years ago, and the other on her right foot 2 months ago. Three years ago, bloody diarrhea and abdominal pain developed, were investigated with colonoscopy. Then, a diagnosis of ulcerative colitis (UC) was made. Her symptoms resolved after administering sulfasalazine 2.0 g/day, and she has been in clinical remission for the past 30 months. Even though she had no recent gastrointestinal symptoms such as diarrhea, abdominal pain, or hematochezia, her skin ulcers had not been healed.
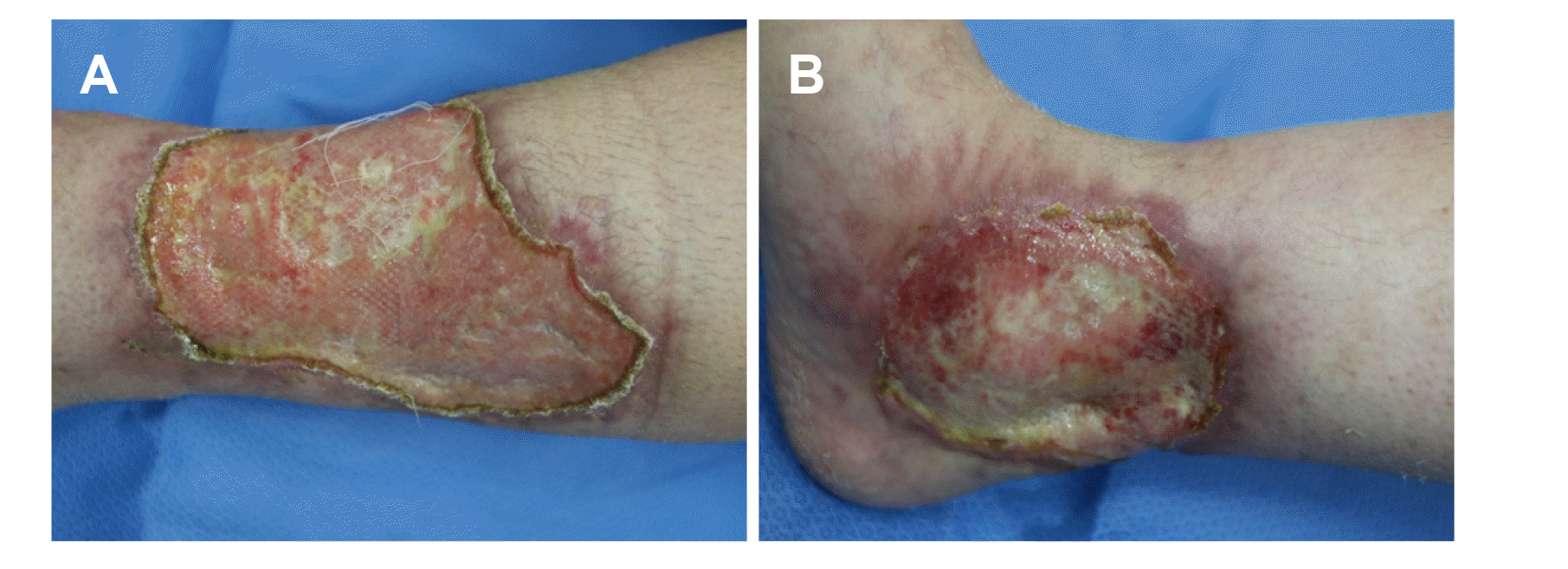
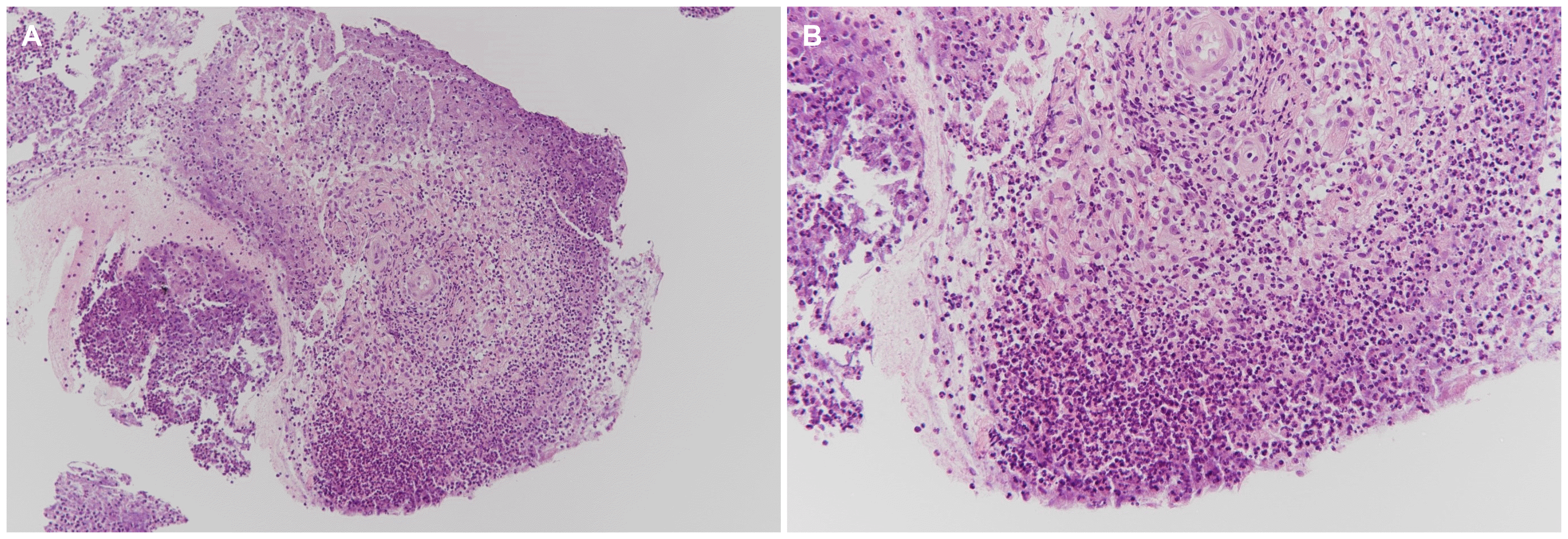
At the time of the hospital visit, her skin lesions had progressively worsened for 6 months without any gastrointestinal symptoms (a partial Mayo score of 0). Previously she had been treated with prednisolone 60 mg/day for 1 year at an outside hospital, but her skin ulcers were not improved and she had experienced weight gain of 15 kg. Therefore, she stopped taking the steroid medications. Her vital signs were within normal limits. The physical examination revealed an ulcer measuring 5×8 cm over the lower third of her left leg and the other measuring 3×3 cm over her right ankle. Gross examination of the ulcers revealed that they were well defined with elevated reddish borders and undermined edges; the bases were covered with necrotic skin and purulent discharge (Fig. 1). Pathogenic bacteria were not detected in the exudates. Histopathological examination of a skin biopsy revealed inflammatory necrotic tissue with several neutrophils (Fig. 2). The laboratory investigations on presentation revealed a white blood cell count of 8,200/mm3, a hemoglobin concentration of 9.3 g/dL, a platelet count of 272,000/mm3, an erythrocyte sedimentation rate of 82 mm/hr (normal: 0-20 mm/hr) and a CRP level of 0.3 mg/dL (normal: 0.0-0.5 mg/dL). Colonoscopy revealed mucosal healing with diffuse scarring and multiple inflammatory pseudo-polyps throughout the colon. No active ulcers or signs of inflammation were noted.
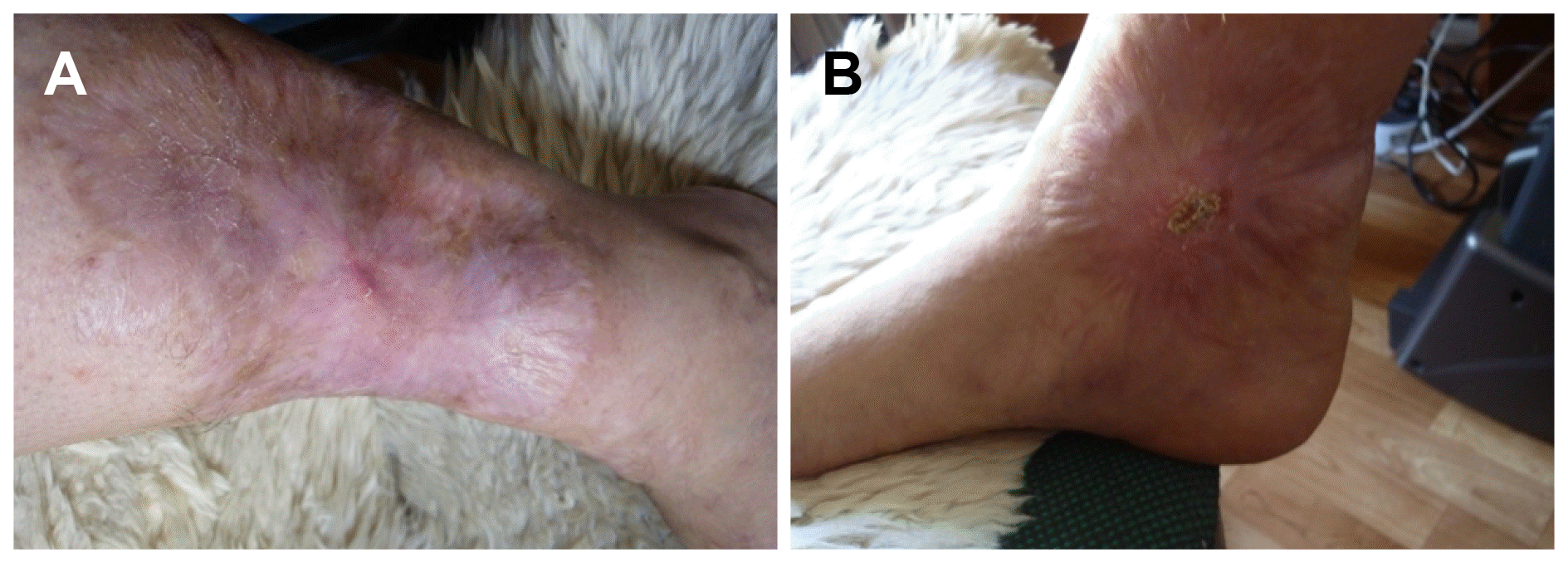
Subsequently, the ulcers on her legs were diagnosed as pyoderma gangrenosum (PG) as an extraintestinal manifestation (EIM) of UC. Because she had no improvement and she had experienced side effect with the previous steroid medication, treatment with azathioprine monotherapy was initiated. We started azathioprine at 25 mg/day for the first 2 weeks, and gradually increased the medication up to 50 mg/day for the next 2 weeks, 100 mg/day for the second month and then 150 mg/day for the third month. The ulcers demonstrated no change in the first month; however, they had healed by approximately 40% 2 months later. The granulation tissue over the ulcers was healthy, and blood vessels and skin had adequately developed. The ulcers were almost completely healed after 1 year of therapy (Fig. 3). She is currently doing well with azathioprine monotherapy without any side effects due to the medications or any complications of UC for 24 months.
Inflammatory bowel disease (IBD) is associated with various EIMs. Previous studies have proven that some EIMs, such as erythema nodosum or sweat syndrome, are associated with disease activity of IBD, but other EIMs, such as PG or uveitis, are not related to intestinal inflammation.1 The prevalence of EIMs in patients with IBD has not been extensively studied. However, in a few studies, 6-40% of patients with IBD had developed more than one EIM.1,2 Up to 15% of patients with IBD develop cutaneous lesions, and the most common cutaneous lesions are erythema nodosum and PG. In one cohort study of 2,402 patients with IBD, PG was detected in only 0.75% of the patients.3
PG is a chronic ulcerative skin disease that usually develops in the early stages of the onset of UC, with the appearance of erythematous and indurated skin lesions that evolve into ulcers with well-defined borders. Such an ulcer begins as a small papule that rapidly grows, develops necrosis, and becomes an enlarged ulcer, which is surrounded by a purple rim and bright red erythema. PG can occur on the lower limbs, as in the case presented here, or it can also affect any other sites of the body, including hands, back, neck and breasts.4,5 The exact pathogenesis of PG is still unknown. In our presented case, inflammatory necrotic tissue with diffuse infiltration of neutrophils was visualized on a skin biopsy. However, it is unclear if primary neutrophil dysfunction plays a role in pathogenesis of PG or if the action of neutrophils is due to an abnormal immune system. It is believed that the pathogenesis of PG may include neutrophil dysfunction, abnormal inflammatory cytokines and/or an abnormal immune system together with combined genetic factors.6 Therefore, systemic and local inflammation should be medically addressed for the effective long-term management of PG.6,7
The management of PG includes local wound care in conjunction with systemic therapy with immunomodulating agents.8 Topical pharmacological treatment, dressings, and injections of corticosteroids can be tried in mild cases. Some of the systemic immunomodulating agents that may be used are corticosteroids, azathioprine, 6-mercaptopurine, cyclosporine, or biological therapy.9 Furthermore, infliximab has been reported to be successful when treating cases of severe or refractory PG.10 In our presented case, the patient’s multiple ulcers of PG completely resolved with azathioprine monotherapy without any complications. Few reports have demonstrated the efficacy of azathioprine monotherapy for treating PG. Azathioprine has generally been used as a second line treatment for moderate or severe UC that is uncontrolled with steroids or maintenance therapy.11 Although the exact mechanism of action of azathioprine is unknown, azathioprine is thought to suppress the proliferation of T and B lymphocytes, and thereby, reduce the production of cytotoxic T cells and plasma cells.12 In the aforementioned case, we found that azathioprine may have a role in the direct treatment of PG as well. The therapeutic role of azathioprine has been previously established in the treatment of PG secondary to mixed connective tissue disorders.13 Thus, our case report may add a new and effective option for treating PG, and especially when PG is associated with UC. There is controversy about the predictive role of PG as a herald of worsening UC. The presence of PG and its severity were suggestive of the extent of gastrointestinal involvement and an exacerbation of the underlying IBD in some previous reports.4,14 However, in our presented case, PG was independent of the IBD activity and the PG developed without any preceding bowel symptoms.
In conclusion, PG may be a complication of inactive UC, and PG may be independent of the activity of bowel inflammation and the further development of preceding bowel symptoms. PG as a complication of UC can be successfully treated with azathioprine monotherapy. A crafty physician should consider administering azathioprine for treating PG combined with IBD.
Go to : 
REFERENCES
1. Bernstein CN, Blanchard JF, Rawsthorne P, Yu N. 2001; The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 96:1116–1122. DOI: 10.1111/j.1572-0241.2001.03756.x. PMID: 11316157.

2. Ricart E, Panaccione R, Loftus EV Jr, et al. 2004; Autoimmune disorders and extraintestinal manifestations in first-degree familial and sporadic inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 10:207–214. DOI: 10.1097/00054725-200405000-00005. PMID: 15290913.
3. Farhi D, Cosnes J, Zizi N, et al. 2008; Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 87:281–293. DOI: 10.1097/MD.0b013e318187cc9c. PMID: 18794711.
4. Brooklyn T, Dunnill G, Probert C. 2006; Diagnosis and treatment of pyoderma gangrenosum. BMJ. 333:181–184. DOI: 10.1136/bmj.333.7560.181. PMID: 16858047. PMCID: PMC1513476.

5. Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. 2009; Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 23:1008–1017. DOI: 10.1111/j.1468-3083.2009.03199.x. PMID: 19470075.

6. Braswell SF, Kostopoulos TC, Ortega-Loayza AG. 2015; Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 73:691–698. DOI: 10.1016/j.jaad.2015.06.021. PMID: 26253362.

7. Adachi Y, Kindzelskii AL, Cookingham G, et al. 1998; Aberrant neutrophil trafficking and metabolic oscillations in severe pyoderma gangrenosum. J Invest Dermatol. 111:259–268. DOI: 10.1046/j.1523-1747.1998.00311.x. PMID: 9699727.

8. Ahronowitz I, Harp J, Shinkai K. 2012; Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 13:191–211. DOI: 10.2165/11595240-000000000-00000. PMID: 22356259.
9. Ehling A, Karrer S, Klebl F, Schäffler A, Müller-Ladner U. 2004; Therapeutic management of pyoderma gangrenosum. Arthritis Rheum. 50:3076–3084. DOI: 10.1002/art.20559. PMID: 15476233.

10. Barrie A, Regueiro M. 2007; Biologic therapy in the management of extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 13:1424–1429. DOI: 10.1002/ibd.20196. PMID: 17567879.

11. Duffill MB. 1994; Cyclosporine, azathioprine and local therapy for pyoderma gangrenosum. Australas J Dermatol. 35:15–18. DOI: 10.1111/j.1440-0960.1994.tb01793.x. PMID: 7998894.

12. Nielsen OH, Vainer B, Rask-Madsen J. 2001; Review article: the treatment of inflammatory bowel disease with 6-mercaptopurine or azathioprine. Aliment Pharmacol Ther. 15:1699–1708. DOI: 10.1046/j.1365-2036.2001.01102.x. PMID: 11683683.
13. Sardar P, Guha P, Das NK, et al. 2011; Ulcerative pyoderma gangrenosum in mixed connective tissue disorder: a rare association and role of azathioprine in the management. Indian J Dermatol. 56:600–602. DOI: 10.4103/0019-5154.87172. PMID: 22121294. PMCID: PMC3221239.

14. Tusnádi A, Uhrin K, Bényei M, Krasznai G. 1994; Ulcerative colitis associated with pyoderma gangrenosum. Orv Hetil. 135:917–919. PMID: 7909938.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download