This article has been
cited by other articles in ScienceCentral.
Abstract
A case of acute pancreatitis associated with hypercalcemia resulting from a newly diagnosed multiple myeloma is reported. There have been several prior reports of hypercalcemia-induced pancreatitis in patients with multiple myeloma, but very few showed the following two aspects: pancreatitis as the first manifestation of hematologic malignancy and recovery after serum calcium levels fell into normal range. When clinicians encounter a patient with hypercalcemic status and acute pancreatitis without any known etiology of pancreatitis, multiple myeloma as a root cause of pancreatitis should be considered.
Go to :

Keywords: Pancreatitis, Hypercalcemia, Multiple myeloma
INTRODUCTION
Although gallstones and chronic alcohol consumption are the most common causes of acute pancreatitis,
1 more than one-third of all acute pancreatitis cases are classified as idiopathic. However, before making a final diagnosis, other less frequent etiologies such as autoimmunity, hypertriglyceridemia, medications, or hypercalcemia should be ruled out.
1,2 Hypercalcemia could induce acute pancreatitis, but only a few cases have been reported as hypercalcemic pancreatitis caused by multiple myeloma itself. Here, we report a case of acute pancreatitis in an elderly male, which was induced by hypercalcemia as an initial presentation of multiple myeloma.
Go to :

CASE REPORT
A 76-year-old man was referred to our institute with diffuse abdominal pain. He had a medical history of hypertension and benign prostate hyperplasia for 20 years. He had not taken any other medication that could cause acute pancreatitis. Moreover, he did not have a history of pancreatitis. He was an ex-smoker who had smoked 30 pack-years and a current drinker consuming about 50 g of alcohol per week.
Pain started 2 weeks ago and had worsened over the past 3 days. At the time of his visit, vital signs were stable, and decreased bowel sound with combined mild tenderness was observed in the periumbilical and epigastric areas. Laboratory findings revealed a white blood cell count of 14,760/mm3 segmented neutrophils content of 75.4%, and hemoglobin count of 9.6 g/dL. BUN and serum creatinine levels were elevated (45.9 mg/dL and 5.0 mg/dL, respectively). Mild hyponatremia and hypokalemia were identified (131.6 mEq/L of sodium and 3.3 mEq/L of potassium). In the liver function tests, serum AST, ALT, total bilirubin, ALP, and GGT were within normal limits (AST 20 U/L, ALT 11 U/L, total bilirubin 0.49 mg/dL, ALP 64 U/L, and GGT 21 U/L). Serum CRP was elevated (10.41 mg/dL, reference value: 0-0.3 mg/dL). Serum triglyceride level was within normal range (67 mg/dL). Autoimmune markers showed a negative fluorescent ANA and IgG4 level of 47.2 mg/L (reference value: 30-2,010 mg/L). Tumor marker levels for CEA and CA 19-9 were normal (1.74 mg/mL and 23.6 U/mL, respectively). The total protein and serum albumin levels were 8.6 g/dL and 2.3 g/dL, respectively, indicating a reversal of the albumin/globulin ratio (0.36). The calcium level was 12.8 mg/dL (as corrected calcium: 14.2 mg/dL) while the phosphorus level was 4.1 mg/dL. Serum amylase and lipase levels were 186 mg/dL (reference value: 22-80 mg/dL) and 259.3 U/L (reference value: less than 60.0 U/L), respectively. The intact parathyroid hormone value decreased to 2.5 pg/mL (reference value: 15-53 pg/mL) while the 25-OH vitamin D level was normal at 34.12 ng/mL (reference value for sufficiency: 32.01-100.00 ng/mL).
Abdomen CT showed a mild edematous pancreatic parenchymal change with peripancreatic haziness, which is compatible with acute interstitial edematous pancreatitis (
Fig. 1). There was no evidence of gallstone or biliary tract dilatation in the CT images, so we excluded gallstone pancreatitis. The skull X-ray image demonstrated multifocal punched-out osteolytic lesions (
Fig. 2).
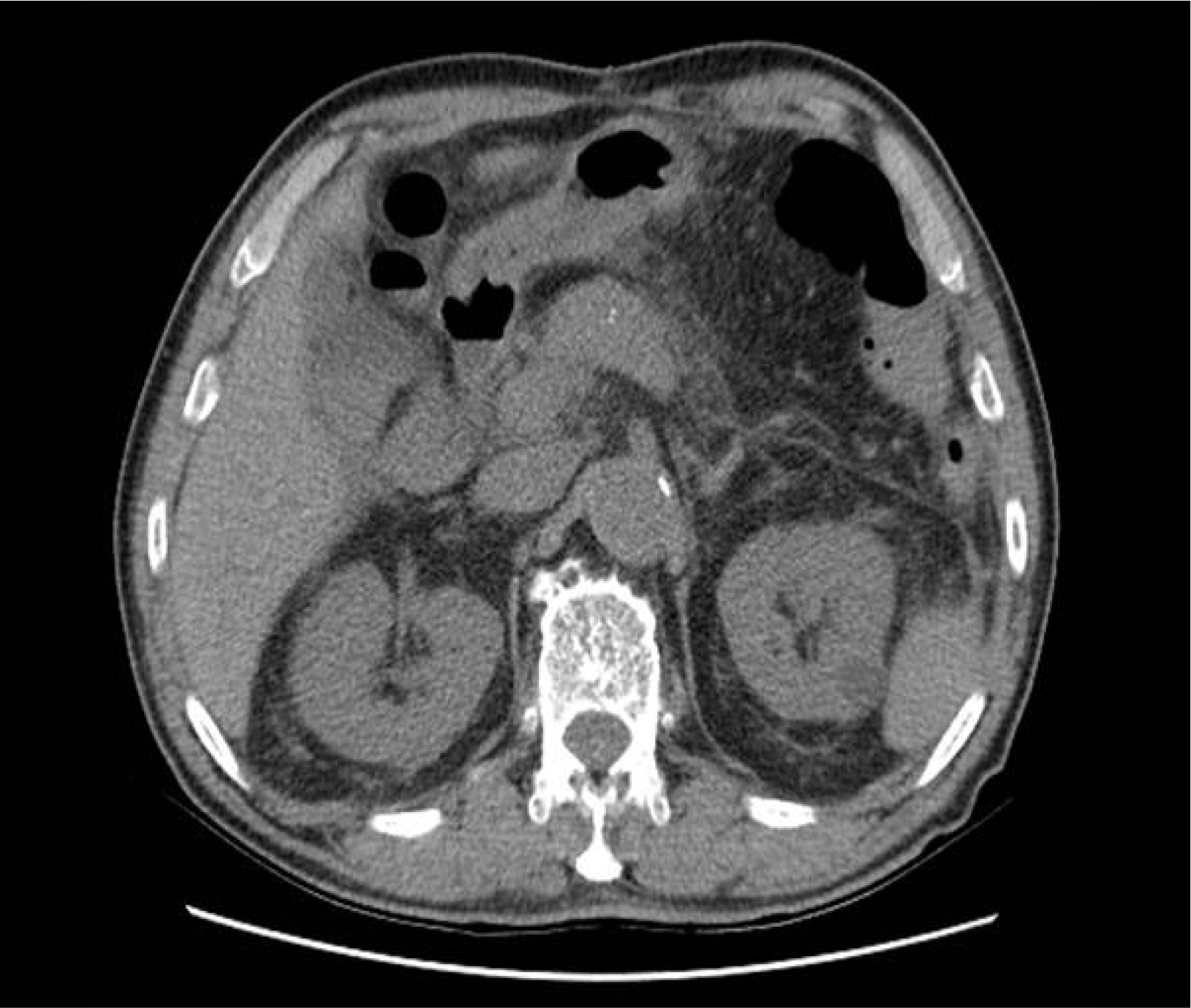 | Fig. 1Computed tomography scan of abdomen (non-enhanced). Mild edematous pancreatic parenchymal change with peripancreatic haziness. 
|
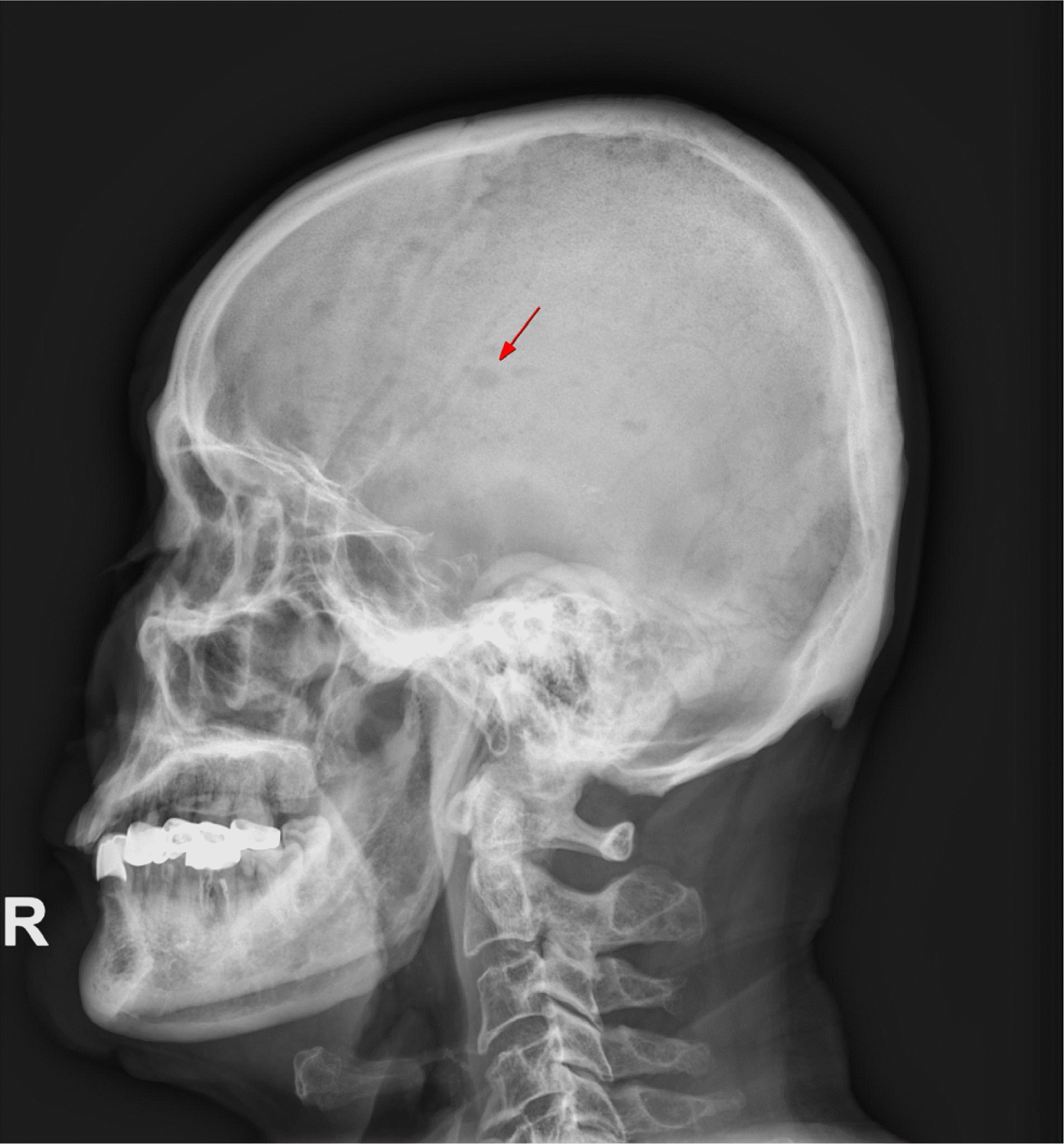 | Fig. 2Skull X-ray image (lateral view). Multiple punched-out osteolytic lesions are scattered in numerous sizes (red arrow). 
|
We carried out protein electrophoresis with immunofixation of both serum and urine, and bone marrow biopsy was planned concurrently. We found an myeloma protein in the gamma region in the electrophoresis (
Fig. 3A) and monoclonal bands corresponding to IgG and κ lanes in the immunofixation (
Fig. 3B), which led us to a diagnosis of monoclonal gammopathy. Bone marrow specimens showed that 60% of all nucleated elements were plasma cell-originated (
Fig. 4A) with CD138 expression in the immunohistochemical stain (
Fig. 4B) and κ clonality in the
in situ hybridization stain. As patients showed 60% plasma cellularity and all hypercalcemia, renal insufficiency, anemia, and bone lesions, our final diagnosis was acute pancreatitis due to hypercalcemia, attributed to multiple myeloma, based on diagnostic criteria provided by the International Myeloma Working Group. For the treatment of pancreatitis, the patient fasted for a few days and then underwent fluid supplementation and intravenous nafamostat mesylate infusion. Hemodialysis was carried out due to worsened dyspnea and progressive renal failure, and serum calcium level started to decrease over 4 weeks, along with gradually normalized serum amylase and lipase levels and improvement of abdominal symptoms (
Fig. 5). After clinical stabilization, the patient started chemotherapy with a lenalidomide-dexamethasone regimen for multiple myeloma, the root cause of acute pancreatitis.
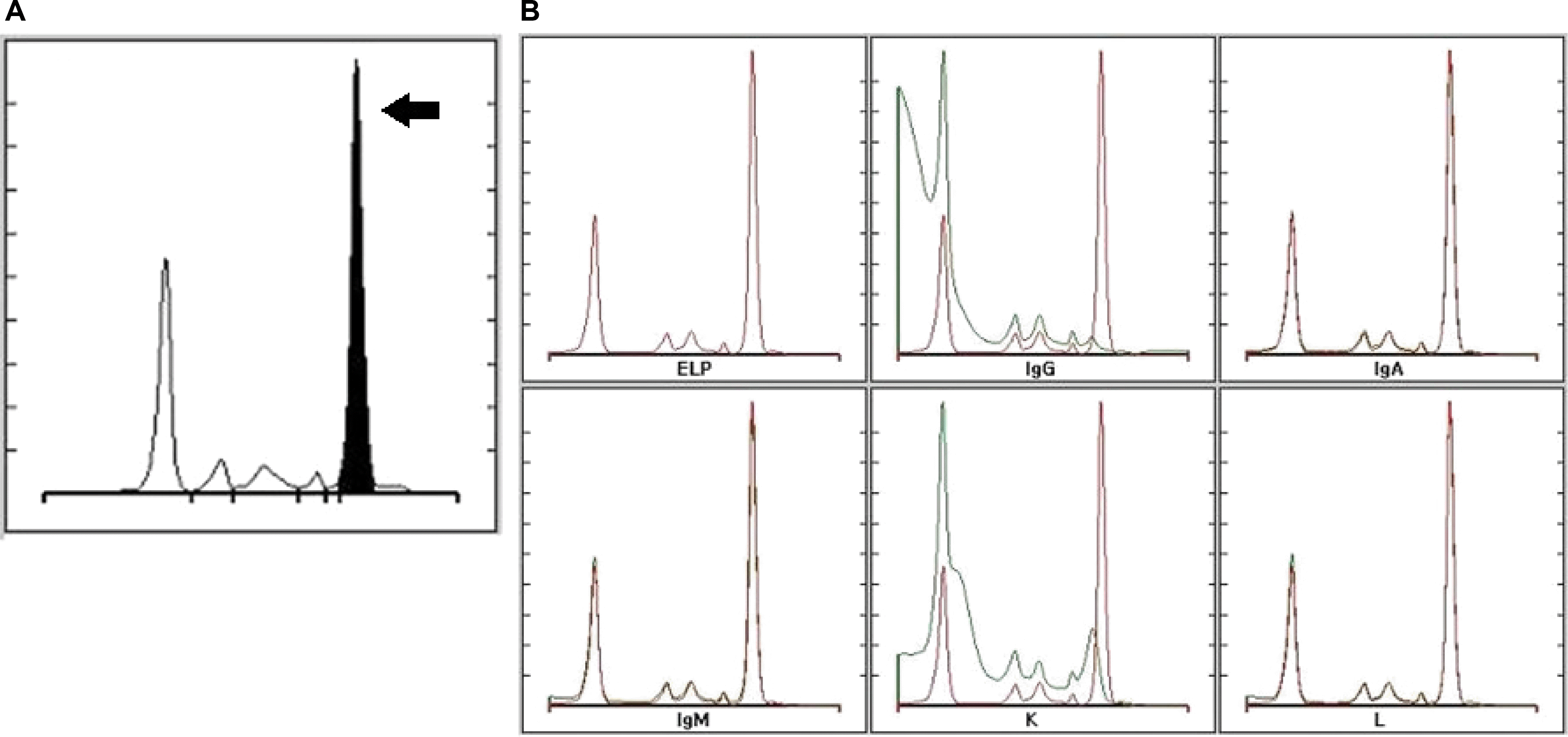 | Fig. 3(A) Serum electrophoresis showing an myeloma protein in the gamma region (black arrow). (B) Serum immunofixation demonstrates monoclonal bands on IgG and κ lanes, which is evidence of monoclonal gammopathy (IgG, κ type). ELP, electrophoresis; Ig, immunoglobulin; κ, kappa; L, lambda. 
|
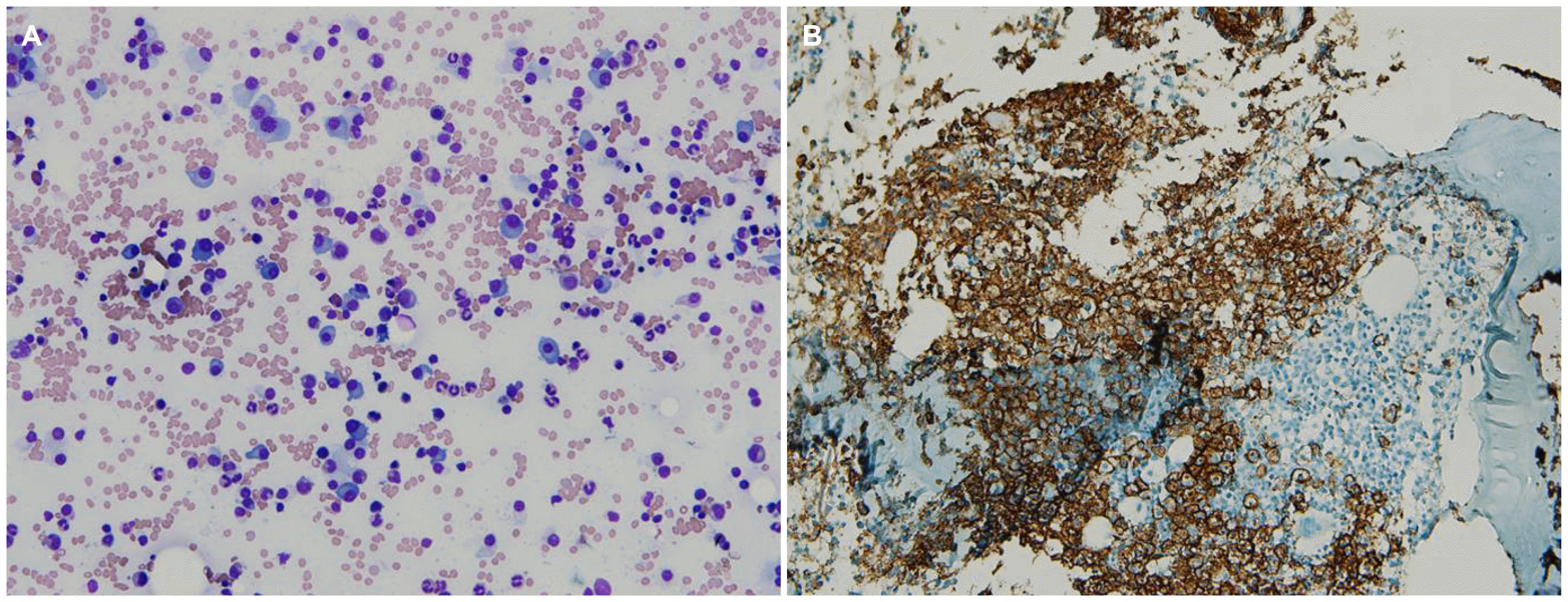 | Fig. 4(A) Microscopic finding of bone marrow shows that about 60% of nucleated elements are plasma cells, which are oval-shaped with a round eccentric nucleus and abundant basophilic cytoplasm (Giemsa stain, ×200). (B) Discrete plasma cell mass displaced normal marrow tissue, expressing CD138 on the immunohistochemical stain (CD138, ×100). 
|
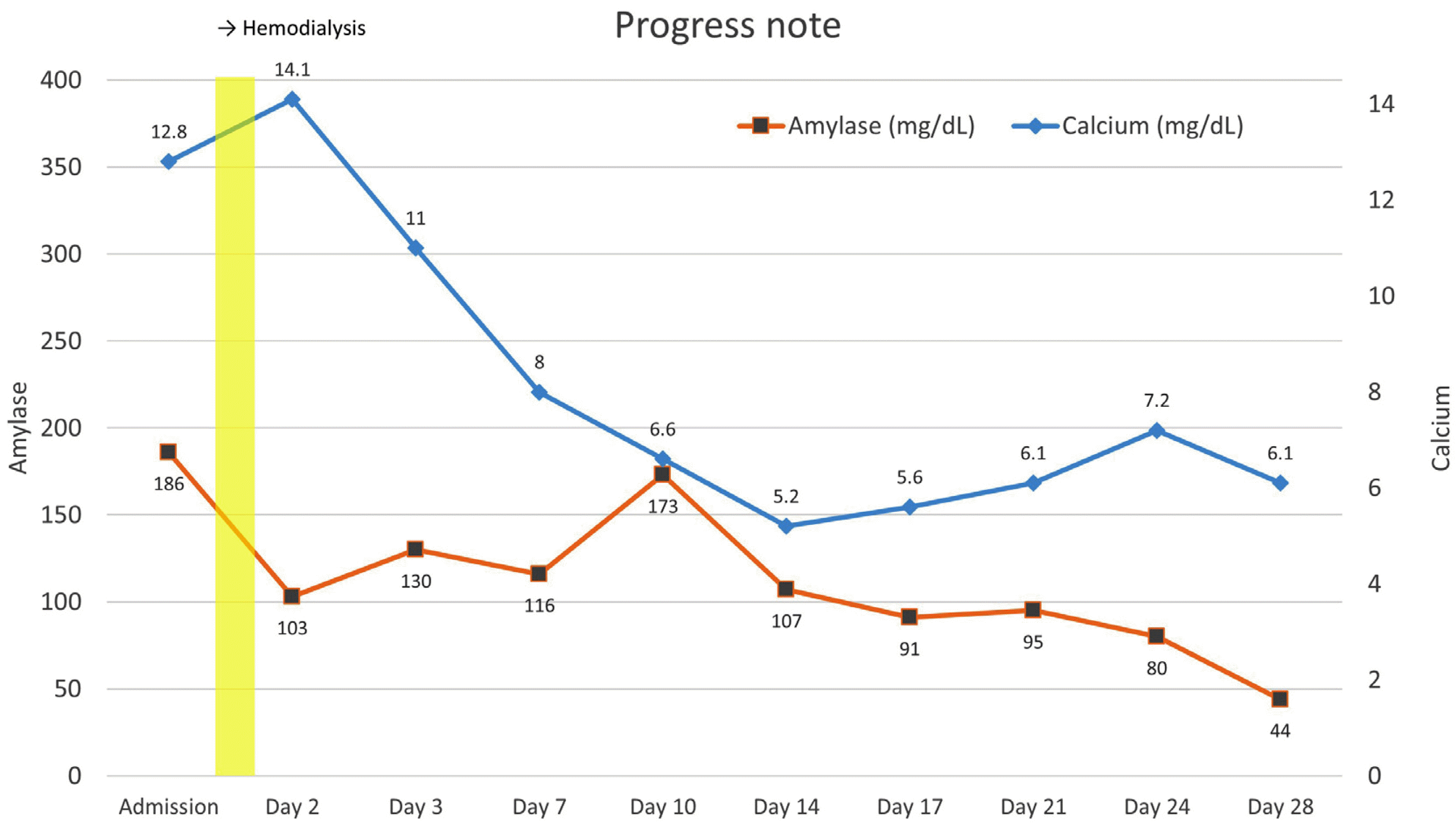 | Fig. 5Changes in serum calcium and amylase level during hospitalization. After hemodialysis, serum amylase levels decreased, as calcium levels had been normalized. 
|
Go to :

DISCUSSION
In our report, we describe a case of acute pancreatitis accompanied with abdominal pain. The patient was initially accompanied by hypercalcemia, albumin/globulin ratio inversion, and anemia, which led to a diagnosis of multiple myeloma. Other causes of pancreatitis such as gallstones, alcohol consumption, and hypertriglyceridemia were excluded through history taking, CT images, and laboratory data. The normalization of serum calcium levels during treatment resulted in clinical improvements of concurrent pancreatitis. This is a rare case in which pancreatitis was the first manifestation of myeloma and was relieved by the correction of hypercalcemia.
Acute pancreatitis is a disease caused by inflammatory reaction of the pancreas parenchyma, and it has been associated with various predisposing factors. According to systematic reviews, its incidence and impact on society has been growing over the last several decades.
3 Nowadays, acute pancreatitis is one of the most frequent gastrointestinal diseases requiring hospitalization in the United States.
4 Hypercalcemia can cause acute pancreatitis, with a reported prevalence of 1.5-8%,
5 but is mostly related to hyperparathyroidism. However, rare cases are caused by secondary hypercalcemia due to malignant disease and so on. The mechanism of pancreatitis attack is thought to be characterized by the shutting down of the pancreatic canaliculi with calcium deposits and by elevation of intracellular trypsinogen enzymatic activity in the pancreas acini.
6 Although hypercalcemia-induced pancreatitis is mainly associated with elevated parathyroid hormone activity due to parathyroid adenoma or carcinoma, paraneoplastic syndromes accompanied by various metastatic cancers, or even milk-alkali syndrome, cases connected with multiple myeloma are uncommon.
Renal failure, anemia, osteolytic lesions, and hypercalcemia are frequently encountered clinical manifestations of multiple myeloma. As for hypercalcemia, about 21% of newly diagnosed symptomatic myeloma patients show elevated serum calcium levels.
7 The mechanism of hypercalcemia in multiple myeloma is complex. Various cytokines produced by myeloma cells induce osteoclast actions, and the total amount of calcium in serum can be elevated as a consequence.
8 Multiple myeloma is diagnosed as manifestation of myeloma caused by hypercalcemia, but pancreatitis develops during treatment of multiple myeloma.
9 The literature also has reported myeloma in younger patients with pancreatitis, but the progress and therapeutic results related to pancreatitis after management of hypercalcemia itself have not been reported.
10,11
In summary, we report a rare case of acute pancreatitis secondary to hypercalcemia in patients who came to the clinic for abdominal pain and were diagnosed with multiple myeloma. The association between pancreatitis and plasma cell-proliferating disorders may be easy to be overlooked due to its rarity. Thus, alertness for diagnosis will be needed when dealing with pancreatitis without a common cause. Clinicians should keep in mind that acute pancreatitis caused by hypercalcemia is one of the initial by-products of multiple myeloma progression.
Go to :







 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download