Abstract
Autosomal dominant chronic mucocutaneous candidiasis (AD-CMC) is a subtype of CMC caused by gain-of-function (GOF) mutation of the signal transducer and the activator of transcription 1 (STAT1) protein. GOF mutation of STAT1 disrupts Th17 cell differentiation and causes susceptibility to candida infection in mucous membranes. Although genetic testing is crucial to diagnose AD-CMC, a simple and fast diagnostic tool is required for the management and reduction of complications associated with infection. Flow cytometry (FCM) is suggested for the measurement of intracellular phosphorylated STAT1 (pSTAT1) in a stimulated status. Here, we report the application of FCM to show the activation status of STAT signaling in a 24-year-old female patient diagnosed with AD-CMC. Compared to the controls, the patient’s T cells showed increased levels of pSTAT1 after stimulation by interferon-γ and lesser extent of inhibition caused by an inhibitor. To the best of our knowledge, this is the first evaluation of the usefulness of FCM as an alternative diagnostic and monitoring tool of GOF STAT1 in Korea.
Go to : 
초록
상염색체 우성 만성점막피부칸디다증(autosomal dominant chronic mucocutaneous candidiasis, AD-CMC)는 만성점막피부칸디다증의 아형 중 하나로 signal transducer and activator of transcription 1 (STAT1) 단백의 기능획득(gain-of-function, GOF) 돌연변이로 인해 나타난다. STAT1단백의 GOF 변이는 Th17 세포의 분화과정에 문제를 야기하고 이로 인해 피부, 점막 등이 캔디다 감염에 취약하게 된다. STAT1단백의 GOF 변이의 확인이AD-CMC 진단에 필수적이지만 감염 합병증을 줄이고 적절한 치료를 위해서는 유전자 검사보다 빠르고 간편한 검사법이 필요하다. 유세포 분석기를 이용하여 자극 후 세포내 인산화 STAT1 단백(pSTAT1)을 측정할 수 있다. 본 보고에서는 AD-CMC로 진단된 24세 여자환자에서 유세포 분석을 통하여 STAT 신호전달 활성화 상태를 보여주었다. 대조군에 비하여 환자의T 림프구에서 감마인터페론으로 자극 후 pSTAT1은 증가되고 저해제를 처리한 후에는 덜 감소하였다. 본 보고는 한국에서 STAT1 GOF 돌연변이를 진단하고 감시하는데 유세포 분석을 이용할 수 있음을 보여준 첫 증례이다.
Go to : 
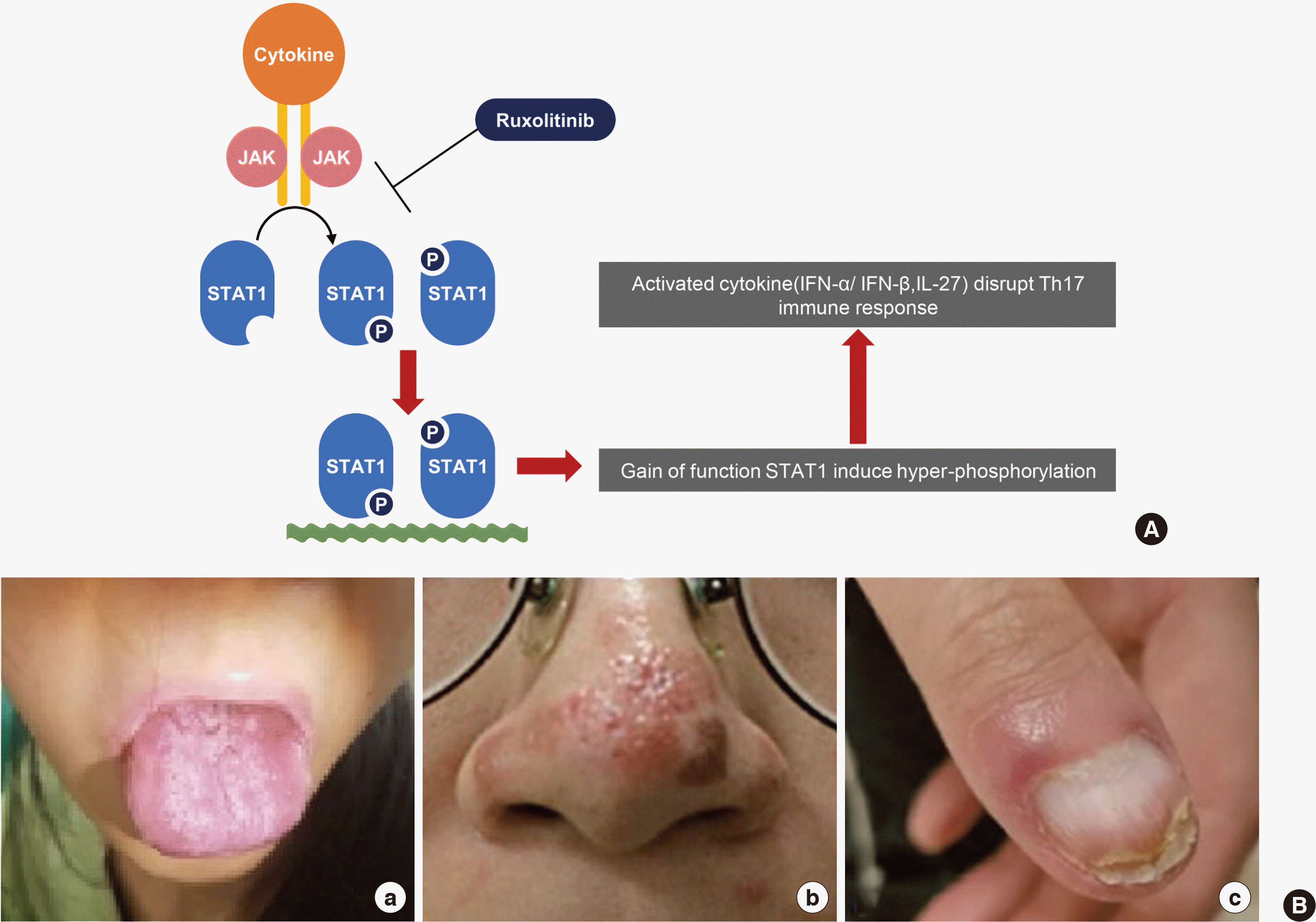
Autosomal dominant chronic mucocutaneous candidiasis (AD-CMC) is a primary immunodeficiency disorder (PID) characterized by a chronic and recurrent mucocutaneous fungal infection [1]. Genetic mutation of the signal transducer and activator of transcription 1 (STAT1) protein has been known to cause AD-CMC [2]. The STAT1 protein is a member of the STAT family regulated by the Janus kinase (JAK), which is translocated to the nucleus and regulates the expression of genes related to STAT signaling and immune system regulation [3]. In particular, gain-of-function (GOF) STAT1 mutation disrupts interferon-γ, interleukin-17, and interleukin-22 signaling, causing defective Th1 and Th17 responses (Fig. 1A) [2, 4].
Although GOF STAT1 mutation can be identified by genetic testing or western blotting, both methods are laborious, time-consuming, and expensive [5]. Recently, flow cytometry (FCM) has been increasingly used to measure intracellular phosphorylated-protein levels. Some studies have suggested that FCM could be used as a diagnostic tool to monitor the phosphorylation of STAT1 (pSTAT1) in addition to genetic testing and western blotting [5, 6]. Here, we report a case of GOF STAT1 mutation in a patient diagnosed with AD-CMC by whole-exome sequencing and present the results of FCM, which are concordant with the sequencing results.
Go to : 
This case report was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2018-07-140). In May 2017, a 24-year-old female patient who suffered from M. massiliense infection with onychomycosis and mucocutaneous candidiasis on her nose and mouth for approximately 10 months at another hospital was transferred to the Samsung Medical Center (Seoul, Korea) (Fig. 1B). She experienced recurrent episodes of oral thrush, sinusitis, and pneumonia from early childhood and was diagnosed with bronchiectasis at the age of 12 and pneumonia caused by Pseudomonas aeruginosa at the age of 18. Based on the presumptive diagnosis of PID, immunological work-up such as the lymphocyte subset analysis, IgG subclass, and autoimmune antibody testing with genetic testing of genes such as the cystic fibrosis transmembrane conductance regulator (CFTR) had been performed. Except for the increased erythrocyte sedimentation rate (64 mm/hr), the patient had normal complete blood count. There were no pathogenic variants of the CFTR gene, and the levels of serum IgG (1,580 mg/dL), IgA (90 mg/dL), IgM (94 mg/dL), and alpha-1-antitrypsin (173.7 mg/dL) were normal. Although the level of the rheumatoid factor (77.9 IU/mL) was increased, the results of the fluorescent antinuclear antibody, antineutrophil cytoplasmic antibody, and extractable nuclear antigen testing were negative. The results of the extractable nuclear antigen testing for SS-A, SS-B, ribonucleoprotein, and Smith were also negative. However, a GOF mutation in the STAT1 protein (NM_007315.3:c.800C>T, p.Ala267Val) was detected by a targeted exome sequencing [7].
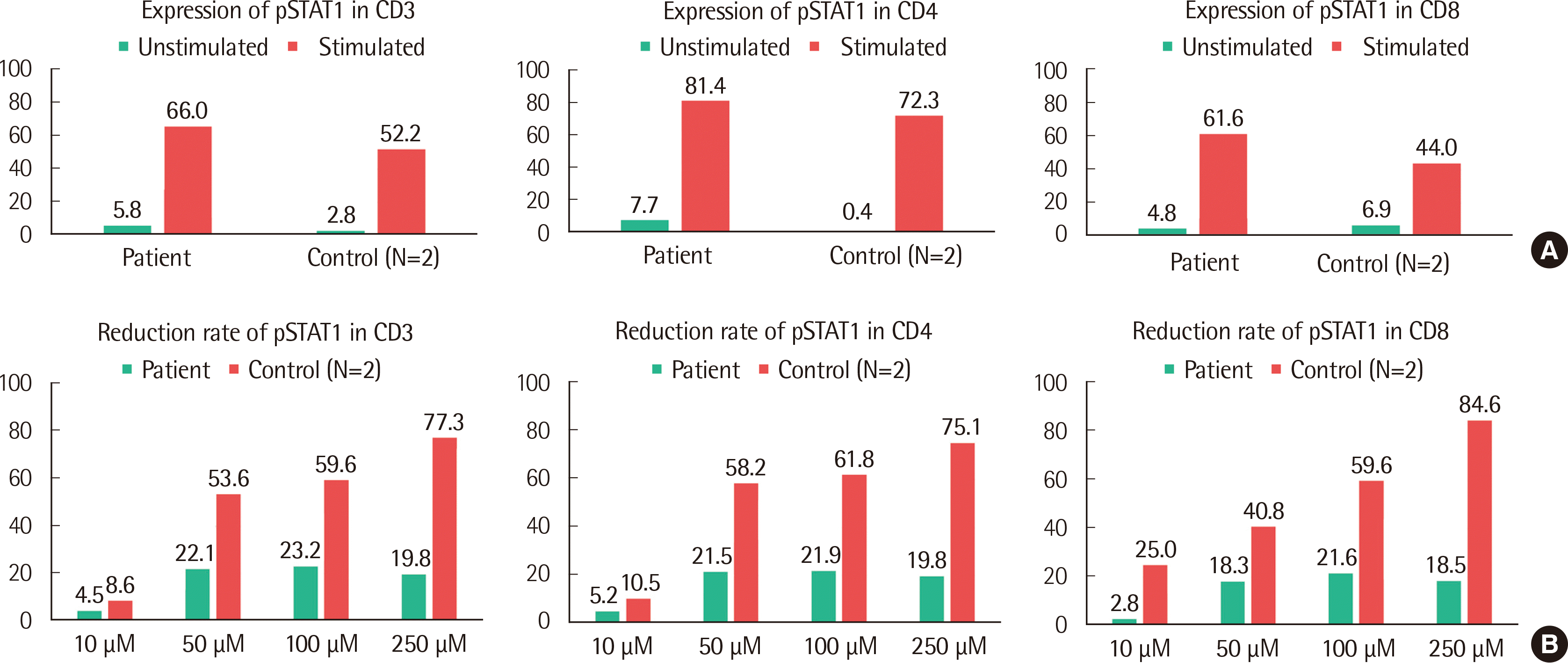
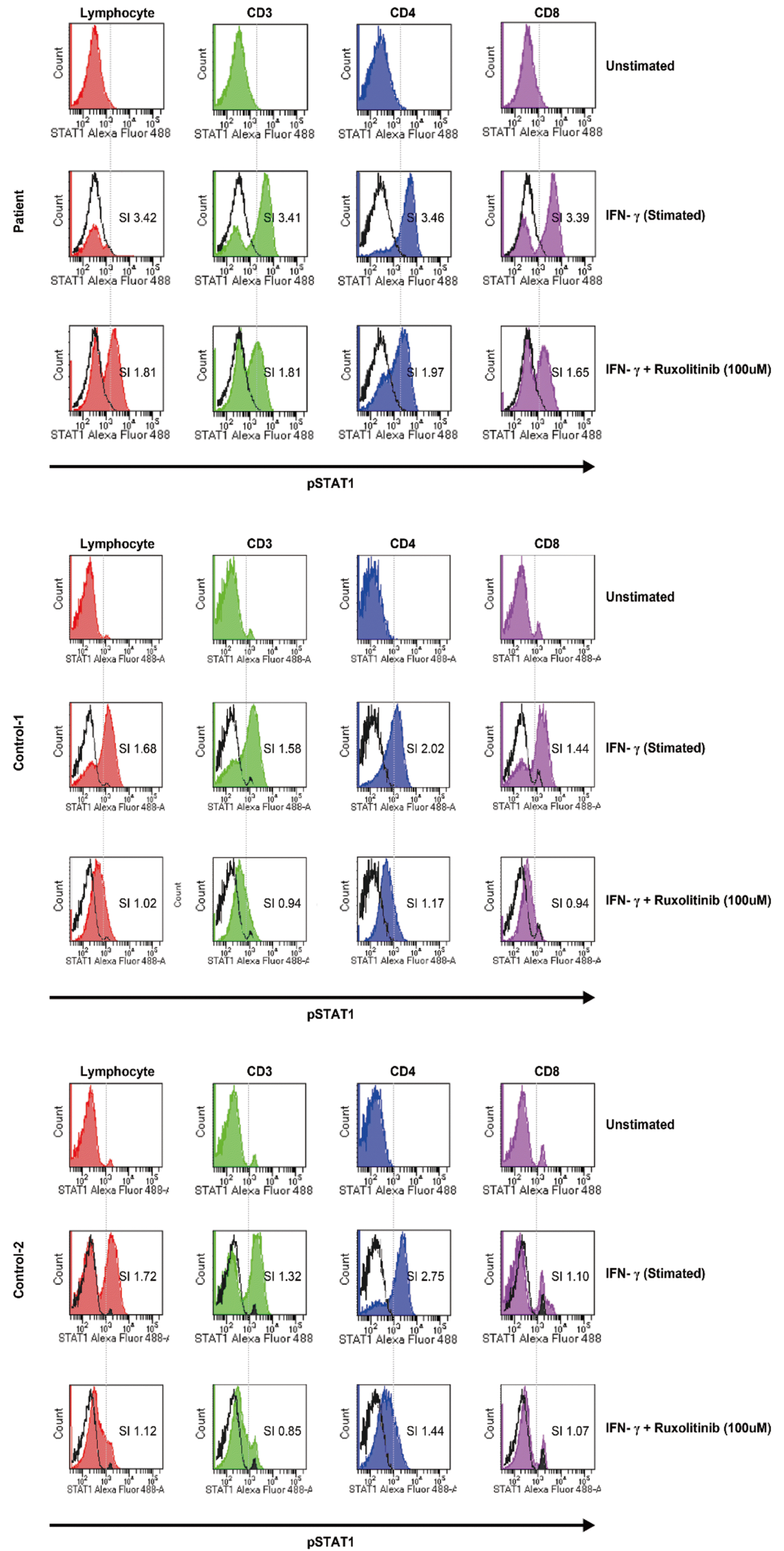
Under the patient’s consent, we investigated the functional consequences of the GOF STAT1 mutation by performing an FCM analysis of the intracellular STAT1 to evaluate whether FCM could be used as a diagnostic and monitoring tool of the STAT1 GOF mutation. Heparin-anticoagulated peripheral blood samples were collected from the patient and normal controls (N=2). Peripheral blood mononuclear cells were acquired by a density gradient centrifugation using Ficoll (General Electric Healthcare Bio-Sciences, Uppsala, Sweden) and stimulated without or with 20 ng/mL interferon-γ (IFN-γ). Ruxolitinib (Novartis Pharmaceutical Corporation, East Hanover, NJ, USA), an inhibitor of JAK1/JAK2, was also added at different concentrations (10 μM, 50 μM, 100 μM, and 250 μM) to observe the inhibition of phosphorylation. The cells were stained with pre-titered Alexa Fluor 488-conjugated pSTAT1 antibody. The stimulation index (SI) was calculated for the gated lymphocytes by dividing the mean fluorescent density (MFI) of stimulated cells by that of the unstimulated cells. Four-color FCM was performed on a FACSCanto Ⅱ apparatus with FACS DIVA (version 8.0) software (all from BD Biosciences, San Jose, USA). When the SI was greater than 2.0, pSTAT1 expression was regarded as significant.
The patient showed a significantly increased MFI, which indicated the expression of pSTAT1 with resistance to the inhibitor compared with the control group. When the CD3+ cells of the patient were stimulated by 20 ng/mL IFN-γ, the SI of pSTAT1 was 3.41 while the mean SI of pSTAT1 was 1.4 in the control group (N=2) (Fig. 2A and Fig. 3). Furthermore, the SI of pSTAT1 was 1.81 when the CD3+ cells of the patient were treated by 100 μM ruxolitinib while the mean SI of the controls was 0.9. The reduction rate of the mean pSTAT1 in the control CD3+ cells was 59.6% and only 23.2% when the patient was treated with 100 μM ruxolitinib (Fig. 2B and Fig. 3, respectively). The results of FCM, which measured the response to IFN-γ without or with ruxolitinib, was in agreement with the pathogenesis of the GOF STAT1 mutation [8]. Although the effect of the GOF STAT1 mutation is not fully understood [1, 9], some studies have recommended ruxolitinib, which inhibits JAK1/JAK2, as a treatment alternative for STAT1 GOF [10-12]. Based on the results of genetic testing and FCM, the patient was treated with ruxolitinib (10 mg, bid) from December 2018 to April 2019. Before she started the medication, the patient had frequent events of abdominal discomfort, oral thrush, and fungal infection even though she was administered 100 mg fluconazole three days a week. After starting ruxolitinib, the patient’s general condition was improved within a week and her oral thrush, along with onychomycosis of a fingernail, was improved after two weeks. During the three-month treatment period, the patient’s general condition including abdominal discomfort was improved. In addition, the patient showed no event of oral thrush, sinusitis, and fungal infection such as candidiasis on her nose or fingernails.
Go to : 
PIDs are inherited disorders of the immune system that have heterogeneous clinical phenotypes [13]. Because of the phenotypic diversity and rare prevalence rate [14], PID diagnosis is challenging and genetic testing is an essential and a promising diagnostic tool for PIDs [15]. Although genetic testing is crucial to diagnose PIDs, fast and anticipative diagnosis of PIDs is also important to manage and reduce complications associated with the infection. In this respect, FCM has recently been suggested as a diagnostic tool for PIDs [5, 6, 16]. STAT1 is a key transcription factor that mediates the IFN-α/β signaling and regulates the immune response via factors such as IFN-γ and interleukin-17 [3], and both loss- and gain-of-function STAT1 mutations have been described in PIDs [9]. In the case of the GOF STAT1 mutation, STAT1 hyper-phosphorylation causes a defect in Th17 cell differentiation, which is crucial for a mucosal antifungal immunity, and induces clinical characteristics of CMC such as an oral thrush and candida infection of the skin and mucous membranes [17]. Thus, the phosphorylation of STAT1 may be observed with FCM and can be used as a trace marker for the treatment response.
In conclusion, we measured the level of pSTAT1 in a patient who was diagnosed with AD-CMC by genetic testing, and the results of FCM analysis were in agreement with the known pathogenesis of the GOF STAT1 mutation. Compared with the normal control group, the patient showed a significant expression of pSTAT1 (greater than two-fold SI) and a decreased reduction rate in response to the inhibitor. Although it is difficult to determine the cut-off value for MFI or SI for the diagnosis of AD-CMC because of the limited number of pSTAT1 measurements performed using FCM worldwide, we suggest that FCM may be used as a simple and fast supplementary diagnostic tool for the GOF STAT1 mutations [5]. In addition to the potential of FCM as a diagnostic tool, FCM-based phosphorylation assays may be useful for monitoring treatment response in patients harboring a GOF or loss-of-function STAT1 mutations [5, 18]. To the best of our knowledge, this is the first report of pSTAT1 measurement using FCM in Korea.
Go to : 
REFERENCES
1. van de Veerdonk FL, Netea MG. 2016; Treatment options for chronic mucocutaneous candidiasis. J Infect. 72:S56–60. DOI: 10.1016/j.jinf.2016.04.023. PMID: 27161991. PMCID: PMC7021088.

2. van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. 2011; STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 365:54–61. DOI: 10.1056/NEJMoa1100102. PMID: 21714643.
3. Shuai K, Liu B. 2003; Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 3:900–11. DOI: 10.1038/nri1226. PMID: 14668806.

4. Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. 2011; Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 208:1635–48. DOI: 10.1084/jem.20110958. PMID: 21727188. PMCID: PMC3149226.
5. Bitar M, Boldt A, Binder S, Borte M, Kentouche K, Borte S, et al. 2017; Flflow cytometric measurement of STAT1 and STAT3 phosphorylation in CD4+ and CD8+ T cells-clinical applications in primary immunodeficiency diagnostics. J Allergy Clin Immunol. 140:1439–41. e9. DOI: 10.1016/j.jaci.2017.05.017. PMID: 28601682.
6. Davies R, Vogelsang P, Jonsson R, Appel S. 2016; An optimized multiplex flow cytometry protocol for the analysis of intracellular signaling in peripheral blood mononuclear cells. J Immunol Methods. 436:58–63. DOI: 10.1016/j.jim.2016.06.007. PMID: 27369043.

7. Huh HJ, Jhun BW, Choi SR, Kim Y-J, Yun SA, Nham E, et al. 2018; Bronchiectasis and recurrent respiratory infections with a de novo STAT1 gain-of-function variant: First case in Korea. Yonsei Med J. 59:1004–7. DOI: 10.3349/ymj.2018.59.8.1004. PMID: 30187709. PMCID: PMC6127433.
8. Smeekens SP, Plantinga TS, van de Veerdonk FL, Heinhuis B, Hoischen A, Joosten LA, et al. 2011; STAT1 hyperphosphorylation and defective IL12R/IL23R signaling underlie defective immunity in autosomal dominant chronic mucocutaneous candidiasis. PLoS One. 6:e29248. DOI: 10.1371/journal.pone.0029248. PMID: 22195034. PMCID: PMC3237610.

9. Boisson-Dupuis S, Kong X-F, Okada S, Cypowyj S, Puel A, Abel L, et al. 2012; Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr Opin Immunol. 24:364–78. DOI: 10.1016/j.coi.2012.04.011. PMID: 22651901. PMCID: PMC3477860.
10. Bloomfield M, Kanderová V, Paračková Z, Vrabcová P, Svatoň M, Froňková E, et al. 2018; Utility of ruxolitinib in a child with chronic mucocutaneous candidiasis caused by a novel STAT1 gain-of-function mutation. J Clin Immunol. 38:589–601. DOI: 10.1007/s10875-018-0519-6. PMID: 29934865.
11. Vargas-Hernández A, Mace EM, Zimmerman O, Zerbe CS, Freeman AF, Rosenzweig S, et al. 2018; Ruxolitinib partially reverses functional natural killer cell deficiency in patients with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations. J Allergy Clin Immunol. 141:2142–55. e5. DOI: 10.1016/j.jaci.2017.08.040. PMID: 29111217. PMCID: PMC5924437.
12. Weinacht KG, Charbonnier Lm, Plant A, Torgerson T, Rosenzweig S, Fleisher T, et al. 2015; Successful therapy of a patient with a novel STAT1 gain of function mutation and life-threatening cytopenias with janus kinase inhibitor ruxolitinib. Blood. 126:3434. DOI: 10.1182/blood.V126.23.3434.3434.
13. Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, et al. 2015; Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency 2015. J Clin Immunol. 35:696–726. DOI: 10.1007/s10875-015-0201-1. PMID: 26482257. PMCID: PMC4659841.
14. Rhim JW, Kim KH, Kim DS, Kim BS, Kim JS, Kim CH, et al. 2012; Prevalence of primary immunodeficiency in Korea. J Korean Med Sci. 27:788–93. DOI: 10.3346/jkms.2012.27.7.788. PMID: 22787376. PMCID: PMC3390729.

15. Rae W, Ward D, Mattocks C, Pengelly RJ, Eren E, Patel SV, et al. 2018; Clinical efficacy of a next-generation sequencing gene panel for primary immunodeficiency diagnostics. Clin Genet. 93:647–55. DOI: 10.1111/cge.13163. PMID: 29077208.

16. Fleisher TA, Madkaikar M, Rosenzweig SD. 2016; Application of flow cytometry in the evaluation of primary immunodeficiencies. Indian J Pediatr. 83:444–9. DOI: 10.1007/s12098-015-2011-0. PMID: 26865168. PMCID: PMC5007620.

17. Eren Akarcan S, Ulusoy Severcan E, Edeer Karaca N, Isik E, Aksu G, Migaud M, et al. 2017; Gain-of-function mutations in STAT1: A recently defined cause for chronic mucocutaneous candidiasis disease mimicking combined immunodeficiencies. Case Reports Immunol. 2017:2846928. DOI: 10.1155/2017/2846928. PMID: 29259832. PMCID: PMC5702932.
18. Vakkila J, Nieminen U, Siitonen S, Turunen U, Halme L, Nuutinen H, et al. 2008; A novel modification of a flow cytometric assay of phosphorylated STAT1 in whole blood monocytes for immunomonitoring of patients on IFN alpha regimen. Scand J Immunol. 67:95–102. DOI: 10.1111/j.1365-3083.2007.02028.x. PMID: 18028288.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download