Previously, additional clonal chromosomal abnormality (CCA) in Philadelphia-positive cells (CCA/Ph
+) has been reported to occur in newly diagnosed CML patients but with poor prognosis. Conversely, unrelated CCAs in Philadelphia-negative cells (CCA/Ph
-) were found to appear in 2 to 17% of CML patients treated with tyrosine kinase inhibitors (TKI), but the clinical impact of CCA/Ph
- has remained debatable [
1-
4]. Among the frequently reported causes of CCA/Ph
- are trisomy 8, monosomy 7/deletion of 6338chromosome 7q (-7/del (7q)), loss of Y chromosome, deletion of 20q (del(20q)), and others. Moreover, -7/del (7q) abnormality has been considered as a warning sign of incidence according to the 2013 European Leukemia Net recommendations [
5].
In this study, we report the case of a CML patient who developed myelodysplastic syndrome (MDS) with monosomy 7 in CCA/Ph- at 196 months of TKI treatment (206 months since CML was diagnosed), after the emergence of del(20q) in CCA/Ph- that transiently existed between 150 and 162 months and then disappeared later. To investigate the time of development of monosomy 7 in CCA/Ph- in the CML patient undergoing TKI treatment, we conducted droplet digital PCR (ddPCR) assay to assess chromosome 7 using the serial bone marrow samples. To the best of our knowledge, this is the first report on monosomy 7 in Philadelphia-negative cells that was confirmed by ddPCR.
The 42-year-old female who was diagnosed with accelerated phase CML exhibited 11% blasts of bone marrow cells based on WHO classification of CML. After receiving 3 months of treatment with interferon and hydroxyurea, she underwent allogeneic stem cell transplantation. However, she exhibited a relapse of CML after 6 months of stem cell transplantation and was thereafter treated with imatinib. She had been suffered from at least one of peripheral blood cytopenia of grade 2 to 4 since the TKI treatment. Peripheral blood cytopenia was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0 [
6]. Philadelphia clones reappeared at 9 months, and were detected again at 36 and 51 months after diagnosis. Thereafter, the therapeutic agent was replaced with a 2nd generation TKI, namely nilotinib (84 months after diagnosis). Bone marrow examinations were performed once every 1–2 years, which showed hypocellular or normocellular marrows without any sign of significant dysplasia even when the transient del (20q) of CCA/Ph
- was detected. After 206 months of diagnosis, she started to exhibit progressive peripheral blood pancytopenia and her bone marrow showed dysplastic features in erythroid and megakaryocytic lineages enabling the diagnosis of MDS (
Fig. 1). Therefore, she was subjected to hypomethylating agent therapy, but her blood cell counts could not be recovered to normal. She was no longer followed up at 224 months of diagnosis.
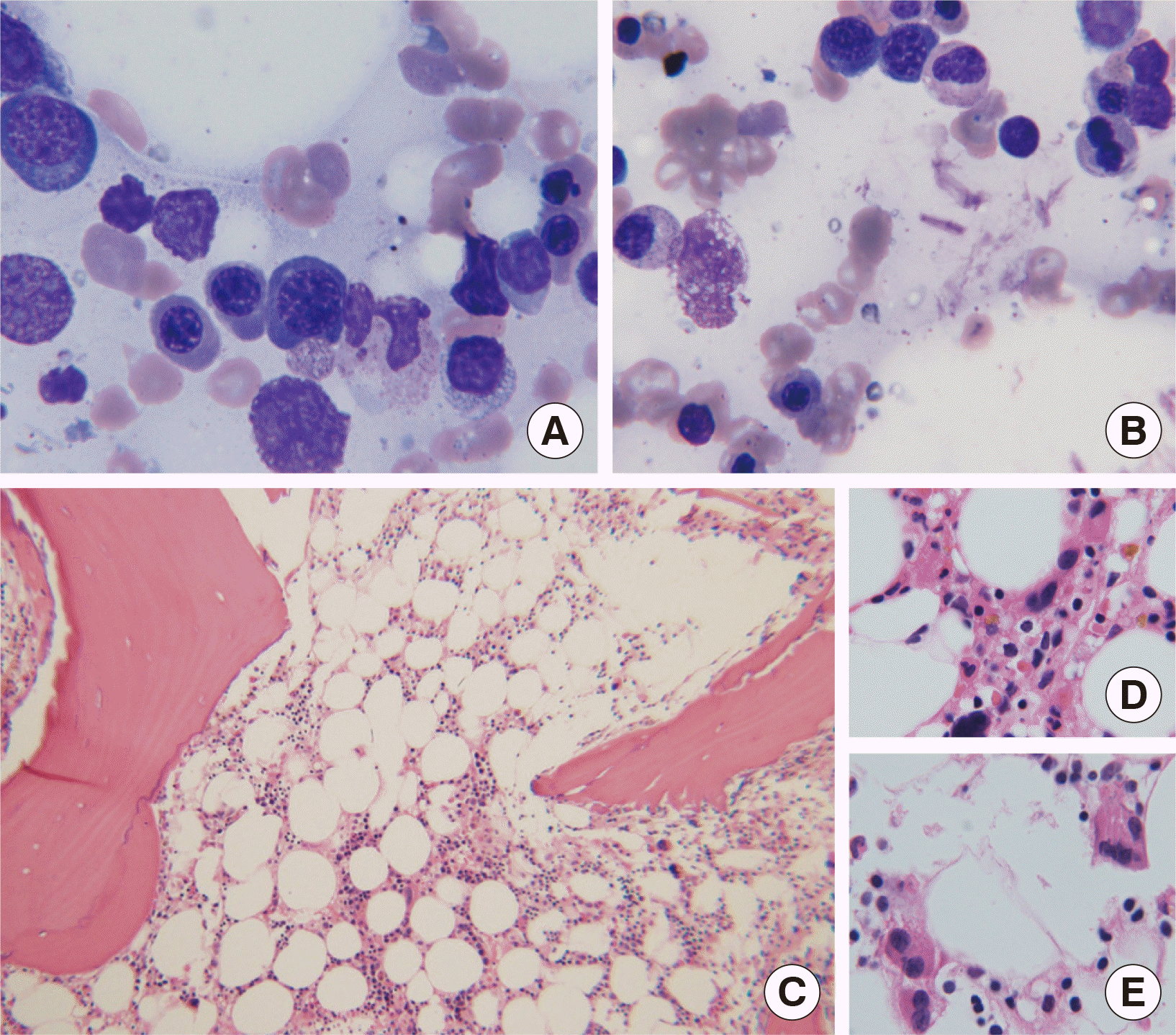 | Fig. 1Bone marrow examination at 206 months since the diagnosis of chronic myeloid leukemia. (A and B) Bone marrow aspirate smears stained with the Wright-Giemsa stain show erythroid dysplasia of nuclear budding and megaloblastoid change, (1,000×). (C) Bone marrow biopsy sections stained with hematoxylin and eosin show hypocellularity (100×) and (D and E) clustered, multinucleated or hypolobated megakaryocytes (1,000×). 
|
Cytogenetic and molecular responses were assessed during the 19-years of follow-up period (
Table 1). Conventional cytogenetic analysis was performed on unstimulated 24- or 48-hour cultures using bone marrow aspirates. Molecular response was assessed by quantitative PCR of
BCR/ABL1 using the commercialized kit (BCR-ABL Mbcr IS (international scale)-MMR Dx kit, IPSOGEN, Marseille, France), according to the manufacturer’s instructions. The molecular responses were presented as major molecular response (MMR,
BCR-ABL1≤0.1% IS) and complete molecular response (CMR, MR4.5 [≥4.5-log reduction in
BCR-ABL1 transcripts from baseline]) [
7]. The ddPCR was performed using the QX200™ Droplet Digital PCR platform (Bio-Rad, Hercules, CA, USA), with a commercially available probe targeting
7q31 (Prime PCR ddPCR Copy Number Assay: MET, human, Bio-RAD) and a reference gene targeting
EIF2C1 (Prime PCR ddPCR Copy Number Assay: EIF2C1, HEX, human, Bio-RAD). Thereafter, the droplet counts were evaluated using the Quanta Soft Analysis Pro Software (Bio-Rad). Peripheral blood samples obtained from 15 healthy individuals were considered as control and showed the result of 2.04±0.12 (mean±SD) for chromosome 7 copy number analyzed by ddPCR.
Table 1
Characteristics of the patient with chronic myeloid leukemia (CML) carrying monosomy 7 in Philadelphia-negative cells
|
Time from diagnosis (months) |
Treatment |
Cytogenetics |
BCR-ABL1 quantitative PCR (IS, %) |
ddPCR for monosomy 7 (Copy number) |
|
|
Type of CCA/Ph-
|
CCA/Ph- (%) |
CyR |
Ph+ (%, No.) |
|
0 |
Diagnosis |
none |
0 |
|
100 (8/8) |
NA |
NA |
|
6 |
3 months post allo-SCT |
none |
0 |
CCyR |
0 (0/15) |
NA |
NA |
|
9 |
6 months post allo-SCT |
none |
0 |
NCyR |
100 (3/3) |
NA |
NA |
|
36 |
imatinib at 26 months |
none |
0 |
PCyR |
10 (2/20) |
NA |
2.12 |
|
51 |
imatinib at 41 months |
none |
0 |
PCyR |
10 (1/9) |
NA |
NA |
|
54 |
imatinib at 44 months |
none |
0 |
CCyR |
0 (0/20) |
NA |
2.06 |
|
60 |
imatinib at 50 months |
none |
0 |
CCyR |
0 (0/20) |
NA |
2.36 |
|
150 |
nilotinib at 66 months |
del (20q) |
40 |
CCyR |
0 (0/37) |
NA |
1.94 |
|
162 |
nilotinib at 78 months |
del (20q) |
5 |
CCyR |
0 (0/40) |
NA |
NA |
|
176 |
nilotinib at 92 months |
none |
0 |
CCyR |
0 (0/5) |
MMR(0.06) |
2.06 |
|
206 |
nilotinib at 122 months |
-7 |
100 |
CCyR, |
0 (0/6) |
CMR (<0.032) |
1.38 |
|
211 |
decitabine at 5 months |
-7 |
85 |
CCyR |
0 (0/20) |
CMR (<0.032) |
1.41*
|
|
223 |
decitabine at 17 months |
-7 |
55 |
CCyR |
0 (0/20) |
CMR (<0.032) |
NA |

At 150 months of TKI treatment for CML, del (20q) in CCA/Ph
- was found to appear that persisted for almost one year. However, later the karyotype returned to normal. At 206 months from CML diagnosis (196 months after the initiation of TKI treatment), monosomy 7 in CCA/Ph
- was found to appear as the only cytogenetic abnormality. The ddPCR showed that the copy number for chromosome 7 had remained normal before the 206th month (
Table 1). At the same time when monosomy 7 was identified by cytogenetic analysis, ddPCR revealed a reduction in the copy number of chromosome 7 (
Table 1).
The exact mechanism leading to the emergence of CCA/Ph
- cells is not fully understood. Also, time of occurrence for CCA/Ph
- cell appearance has not been discovered yet. Some researchers previously suggested that when CML patients are exposed to genomic changes, it leads to the emergence of multiple abnormal clones, including t(9;22) clones, even before the diagnosis of CML [
4,
8,
9]. Therefore, the preexisting CCA/Ph
- clones may not be apparent during the proliferative phase of the Philadelphia-positive clones. However, TKI treatment suppresses the proliferation of Philadelphia-positive clones and thus may allow the CCA/Ph
- clones to emerge. The CCA/Ph
- clones may thus represent a form of genomic instability in patients with CML. The other hypotheses is that CCA/Ph
- may get involved in leukemic process as a result of TKI treatment with evidences of the absence of CCA/Ph
- clones at the time of diagnosis [
1,
10]. TKI targeting
BCR/ABL1 oncoprotein may predispose genomic instability and lead to the emergence of CCA/Ph
- [
1]. Therefore, TKI treatment itself might contribute in the development of CCA/Ph
- clones in patients with CML.
Fluorescence
in situ hybridization (FISH) test is a highly sensitive method when compared to the conventional cytogenetic methods, and thus may allow more frequent discovery of CCA/Ph
-, which could be missed by conventional cytogenetics in TKI treated CML patients [
1]. The sensitivity of ddPCR is known to be higher than that of FISH, as it is able to detect copy number variation at a level of less than 1% of somatic mosaicism [
11]. In this case study, we used ddPCR to assess serial specimens that were obtained from a patient with CML, the monosomy 7 clone in CCA/Ph
- had not been observed until 176 months since CML diagnosis. Our finding suggests that monosomy 7 in CCA/Ph
- might develop as a result of TKI treatment, which is not present initially at the time of CML diagnosis.
Persistent cytopenia during TKI treatment of patients with CML has often been reported, but is rarely associated with disease transformation to MDS, AML, or aplastic anemia [
12-
15]. In this case study, the patient had persistent peripheral blood cytopenia since the beginning of TKI treatment and then, was finally diagnosed with MDS accompanied with newly occurred monosomy 7 in CCA/Ph
- at 206 months from CML diagnosis.
Bone marrow examination is not considered as a prerequisite for disease monitoring in the case of CML [
7]. The clinical significance of CCA/Ph
- has not been well established. Since most cases of CCA/Ph
- are transient and exhibit benign clinical course [
1,
3,
16], routine bone marrow examination is not required. However, recent reports have shown that poor prognosis of certain types of CCA/Ph
-, especially -7/del(7q), increases the risk of disease progression to AML or MDS [
17-
19]. In the present case study, del(20q) in CCA/Ph
- cells occurred during the follow up period and the clinical course remained stable for one year. However, after the disappearance of del(20q), -7 emerged in the CCA/Ph
- cells that later transformed into MDS. Altogether, these findings suggest that persistent cytopenia should be monitored cautiously and may require bone marrow examination for detection of CCA/Ph
- cells.
In summary, this study suggests the emergence of monosomy 7 in CCA/Ph- cells may occur as a result of TKI treatment. Also, it is important to examine the CML patients with persistent cytopenia who undergo TKI treatment using conventional cytogenetic techniques on bone marrow samples to identify possible genetic changes occurring in the CCA/Ph- cells.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download