INTRODUCTION
Mucormycosis is one of the most common fungal infections caused by species belonging to the order Mucorales such as
Mucor and
Rhizopus [
1]. Similar to other invasive fungal infections, mucormycosis can occur in immunocompromised patients with cancer, inherited immunodeficiency, diabetes mellitus, and even organ transplant recipients [
2,
3]. Mucormycosis is difficult to treat due to fast dissemination and low susceptibility to anti-fungal agents, which results in worse outcomes than other common fungal infections, such as aspergillosis [
4]. Peritonitis preceded by gastrointestinal (GI) mucormycosis is very rare, and only a few cases have been reported, which were caused by
Rhizopus microsporus [
5-
7]. We present a case of peritonitis and disseminated mucormycosis caused by
Mucor circinelloides in a patient with nodal marginal zone B-cell lymphoma.
Go to :

CASE REPORT
A 59-year-old man who was diagnosed with nodal marginal zone B-cell lymphoma and received scheduled chemotherapy was hospitalized for one day for high fever (39.5°C) and general weakness. His complete blood counts were as follows: hemoglobin 86 g/L, white blood cells (WBC) 0.11×10
9/L, platelets 36× 10
9/L. He also had a neutropenic fever. After two days, he showed abnormal results of liver function test (LFT), and his other blood tests reports were as follows: aspartate aminotransferase 13,476 IU/L, alanine aminotransferase 4,591 IU/L, total bilirubin 3.37 mg/dL, γ-glutamyl transferase 51 IU/L, and high-sensitivity C-reactive protein 18.80 mg/dL.
Pseudomonas aeruginosa was grown on sputum and blood culture in all of two culture bottles. The galactomannan test (PLATELIA
Aspergillus Ag, Bio-Rad, Marnes-la-Co-quette, France) of serum was positive, and chest computed tomo-graphy revealed multifocal pneumonia combined with pulmonary aspergillosis. He received antibiotics (meropenem, cefepime, and vancomycin) for pneumonia and caspofungin (35 mg daily for two weeks) for aspergillosis. Ten days after showing LFT abnormality, an abdominal computed tomography to evaluate LFT abnormality revealed a large amount of ascites (
Fig. 1A). He was diagnosed with liver failure due to severe septic shock. The laboratory test results of serum were: total protein 47 g/L, albumin 27 g/L, glucose 133 mg/dL, lactate dehydrogenase (LDH) 814 IU/L. Cell counts and other laboratory test results of ascites were: WBC 4.5×10
9/L (neutrophils were 93% of WBC), red blood cells 24.0× 10
9/L, total protein 13 g/L, albumin 7 g/L, glucose 117 mg/dL, LDH 630 IU/L. The serum ascites to albumin gradient was 20 g/L.
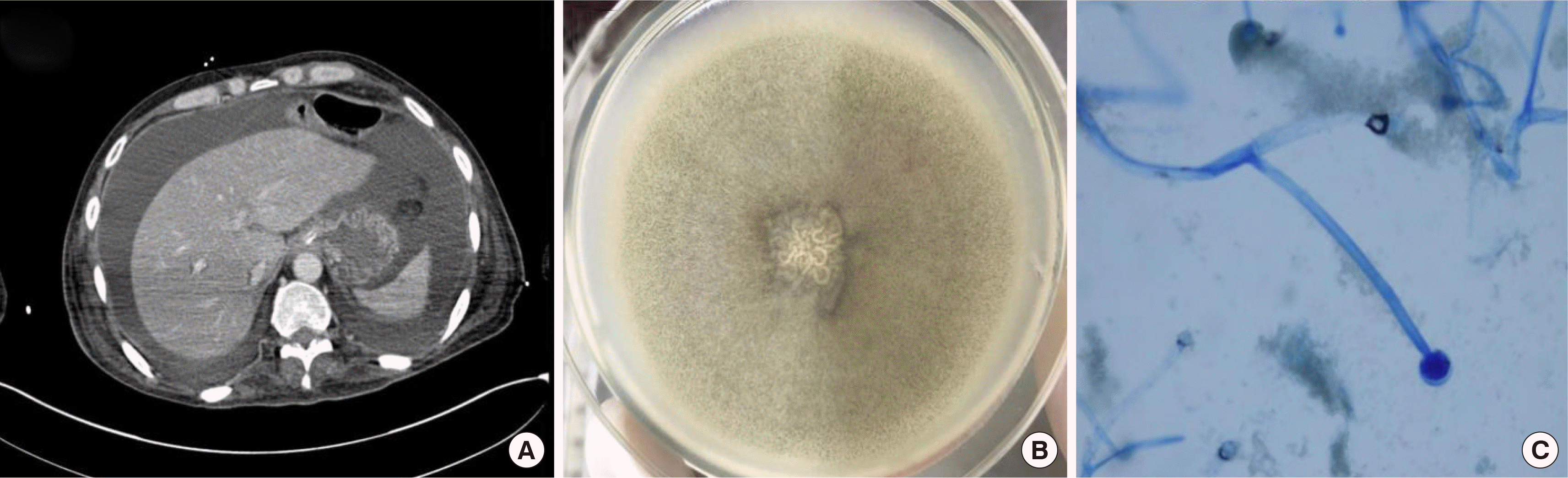 | Fig. 1Computed tomography (CT) scan and identified Mucor circinelloides from the patient. (A) Ascites in abdominal CT. (B) Macroscopic feature on Sabouraud dextrose agar plate. (C) Microscopic features (×200) after lactophenol cotton blue staining. 
|
A specimen of ascites in a blood culture bottle was transferred to laboratory, and cultured on a blood agar plate at 37°C and 5% CO2 atmosphere for 15 hours. Mold grown on blood agar plate was inoculated on a Sabouraud dextrose agar and incubated at 30°C. A uffy, white, cottony growth was observed on Sabouraud dextrose agar at 48 hours (
Fig. 1B). The color turned greyish brown with time, though the part visible from the bottom of the plate remained pale white. Staining with lactophenol cotton blue showed irregular aseptate hyphae branched at obtuse angles without rhizoids. Globose sporangia were filled with round spores and located at the end of long sporangiophores (
Fig. 1C). The macroscopic and microscopic morphologies were consistent with
Mucor species. The (1,3)-β-D-glucan and galactomannan tests of ascites and serum were negative. Only
P. aeruginosa and
Stenotrophomonas maltophilia were seen growing in sputum culture, and no growth was observed on pleural uid culture. After five days, another set of ascites and sputum specimens of the same patient were cultured, and the same mold was isolated.
For accurate identification of the species, the pure culture isolated on Sabouraud dextrose agar was analyzed by using polymerase chain reaction and direct sequencing analysis targeting the internal transcribed spacer (ITS) and the D1/D2 regions. DNA extraction was performed on a MagNA Pure 96 system (Roche Diagnostics, Mannheim, Germany) according to the procedure recommended by the manufacturer. BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) and ABI Prism 3730 Genetic Analyzer (Applied Biosystems) were used for sequencing of the extracted and amplified DNA products. According to the Clinical and Laboratory Standards Institute guidelines (MM18-A) [
8], primer sets of ITS-1/ITS-4 and ITS-5/ITS-4 were used for amplifying ITS, and a primer set of NL-1/NL-4 was used for amplifying the D1/D2 region of ribosomal DNA. For identification, the BLAST search was performed in the GenBank database available at the NCBI website (
http://www.ncbi.nlm.nih.gov). Targeted DNA sequencing confirmed the isolate as
M. circinelloides with 100% identity for both ITS and D1/D2 regions. Though the patient received amphotericin B (300 mg daily) as soon as
Mucor species was identified, he died four days after receiving amphotericin B.
Go to :

DISCUSSION
Mucormycosis is a fatal infection caused by fungi of the order Mucorales. The most common genera that cause mucormycosis are
Rhizopus,
Mucor, and
Lichtheimia (formerly
Absidia and
Mycocladus). Mucormycosis is an opportunistic infection in immunocompromised patients, especially those with hematologic malignancy, severe neutropenia, and diabetes mellitus [
2,
3]. Mucormycosis can cause outbreaks among the patients with risk factors, and the incidence rate of mucormycosis related hospitalizations shows an average every annual increase of >5%. Several outbreaks of mucormycosis were investigated, and all of them were associated with infections in healthcare environments caused by insufficient air filtration and contaminated objects [
9]. We identified
Mucor in only one patient, and the possibility of outbreak seems low.
Unlike other fungal infections, mucormycosis shows rapid progression and angioinvasion, and causes tissue necrosis and dissemination. Mucorales cause invasive infection at various levels including cutaneous, rhinocerebral, pulmonary, GI, and disseminated. In rare cases, mucormycosis can also cause peritonitis, endocarditis, and osteomyelitis [
2,
3].
Several cases of mucormycosis peritonitis were reported, and most of them were related with peritoneal dialysis related mucormycosis [
10-
14]. Only a few cases of peritonitis were preceded by GI mucormycosis, three such reported cases are listed in
Table 1 [
5-
7]: two were related to perforation of sigmoid colon or small intestine caused by
R. microspores, while one was related to perforation of jejunum. The microscopic features of jejunum were consistent with GI mucormycosis; however, the identification of fungal species was not available. After anti-fungal treatment including amphotericin B and voriconazole, all patients died within 47 days. In our case, the patient did not have peritoneal dialysis history, and there was a possibility that GI mucormycosis occurred and proceeded to peritonitis. This is a rare case of peritonitis and disseminated mucormycosis probably preceded by GI mucormycosis caused by
M. circinelloides.
Table 1
Cases of peritonitis preceded by GI mucormycosis
|
Reference |
Country |
Age/sex |
Source of Infection |
Specimen |
Culture Result |
Molecular Identification |
|
Monecke et al. 2006 [5] |
Germany |
62/F |
Perforation of sigmoid colon |
Peritoneal swabs |
Rhizopus spp. |
R. microsporus
|
|
Hyvernat et al. 2010 [6] |
France |
52/F |
Perforation of small intestine |
Intra-abdominal fluid |
Rhizopus spp. |
R. microsporus
|
|
Kumar et al. 2017 [7] |
India |
24/F |
Perforation of jejunum |
Resected segment of jejunum |
NA |
NA |
|
Present case |
Korea |
59/M |
Unknown (probably GI origin) |
Ascites and sputum |
Mucor spp. |
M. circinelloides
|

GI mucormycosis is uncommon, accounting for 0% to 9% of mucormycosis depending on the host conditions, notably 3% in patients with malignancy [
2]. GI mucormycosis can occur after ingestion of foods contaminated with mold, and can proceed to perforation, peritonitis, and sepsis caused by fungal invasion to bowel walls and blood vessels [
3]. In addition, an outbreak of GI mucormycosis has also been reported, which occurred after ingesting yogurts contaminated with mold that were later identified as
M. circinelloides [
15].
So far, there are no available serological biomarkers for diagnosing mucormycosis, such as (1,3)-β-D-glucan and galactomannan, which are used for diagnosing other invasive fungal infection [
4]. The traditional culture method is still important, however, it takes time to isolate and identify a fungus as compared to the rapid progression of Mucorales [
4]. Amphotericin B has been recommended as first-line treatment for mucormycosis [
16,
17]. Posa-conazole and voriconazole are more effective against some Mucorales [
4]. Various Mucorales and even the individual species show variable response to anti-fungal agents [
4]. Therefore, mucormycosis is difficult to diagnose early and to be treated successfully, which results into higher mortality that exceeds 50% despite anti-fungal treatment [
4]. Disseminated mucormycosis especially shows fatal outcome [
3]. In this case, the patient died four days after receiving amphotericin B. He had several risk factors that were prone to fungal infection and its fast dissemination, including hematology malignancy, neutropenic status, and use of antibiotics. Five days after identifying
Mucor species of initial ascites specimen, the same mold was isolated in the sputum specimen. He might have shown GI mucormycosis of
M. circinelloides followed by peritonitis and disseminated mucormycosis in the lung; however, the anti-fungal susceptibility test of the isolate was not performed in this case.
Yang et al. [
18] have reported the identification of Mucorales using molecular methods. But there have been some discrepancies cases between microscopic morphological and molecular identification. Some species of Mucorales cannot be easily identified solely based on the morphology. Therefore, molecular methods are considered to be more reliable and need to be performed for accurate identification of Mucorales [
18]. This case showed concordance between morphological and molecular identification.
In conclusion, we report a rare case of peritonitis and disseminated mucormycosis probably preceded by GI mucormycosis caused by M. circinelloides. Accurate and rapid identification of the mold using molecular methods might be necessary for early treatment in critical and rare cases, and more cases should be clinically evaluated further.
Go to :





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download