Abstract
Pestalotiopsis species are filamentous fungi that are known plant pathogens commonly isolated in tropical and subtropical regions. To the best of our knowledge, this is the first case of human infection caused by Pestalotiopsis mangiferae. An 80-year-old male farmer presented with ocular pain in the right eye. At initial presentation, slit-lamp examination showed a 3.0×2.5 mm-sized epithelial defect in the cornea of the right eye accompanied by corneal thinning. A KOH examination revealed spores, and consequently, treatment with voriconazole, ceftazidime, and moxifloxacin was initiated. One month later, a second KOH examination and fungal culture were performed. The results of the KOH examination indicated the presence of many hyphae, and fungus was isolated from the culture. Molecular identification revealed that the sequence had 100% similarity to P. mangiferae. The patient was treated with therapeutic penetrating keratoplasty. During follow-up in the outpatient clinic, signs of infection were not observed.
Go to : 
초록
Pestalotiopsis 종은 사상형 진균으로 아열대 및 열대 지역에서 일반적으로 분리되는 식물 병원균으로 알려져 있다. 본 증례는 Pestalotiopsis mangiferae에 의해 사람이 감염된 첫 번째 사례이다. 80세 남성 농부가 오른쪽 눈의 안구통증으로 병원을 방문하였다. 슬릿램프검사상으로 오른쪽 눈의 각막은 얇아져 있었고, 3.0×2.5 mm 크기의 상피 결함이 관찰되었다. KOH 검사에서 포자가 관찰되어, 보리코나졸, 세프타지딤 및 목시플록사신으로 치료를 시작하였다. 한 달 후 시행한 KOH 검사에서 많은 균사가 관찰되었고, 배양에서 진균이 분리되었다. 분자검사상으로 P. mangiferae와 100% 일치하는 염기서열임을 확인하였다. 전체층각막이식을 시행하였고, 추적 관찰 동안 감염 징후는 관찰되지 않았다.
Go to : 
Pestalotiopsis species are commonly isolated in subtropical and tropical regions and are associated with living plants. They represent a fungal group known to produce a wide range of bioactive metabolites, such as the anticancer agent taxol [1, 2]. They are common phytopathogens that cause a variety of plant diseases. Pestalotiopsis mangiferae is a plant pathogen that particularly affects Mangifera indica [3]. To the best of our knowledge, this is the ¬rst case of human infection caused by P. mangiferae.
Go to : 
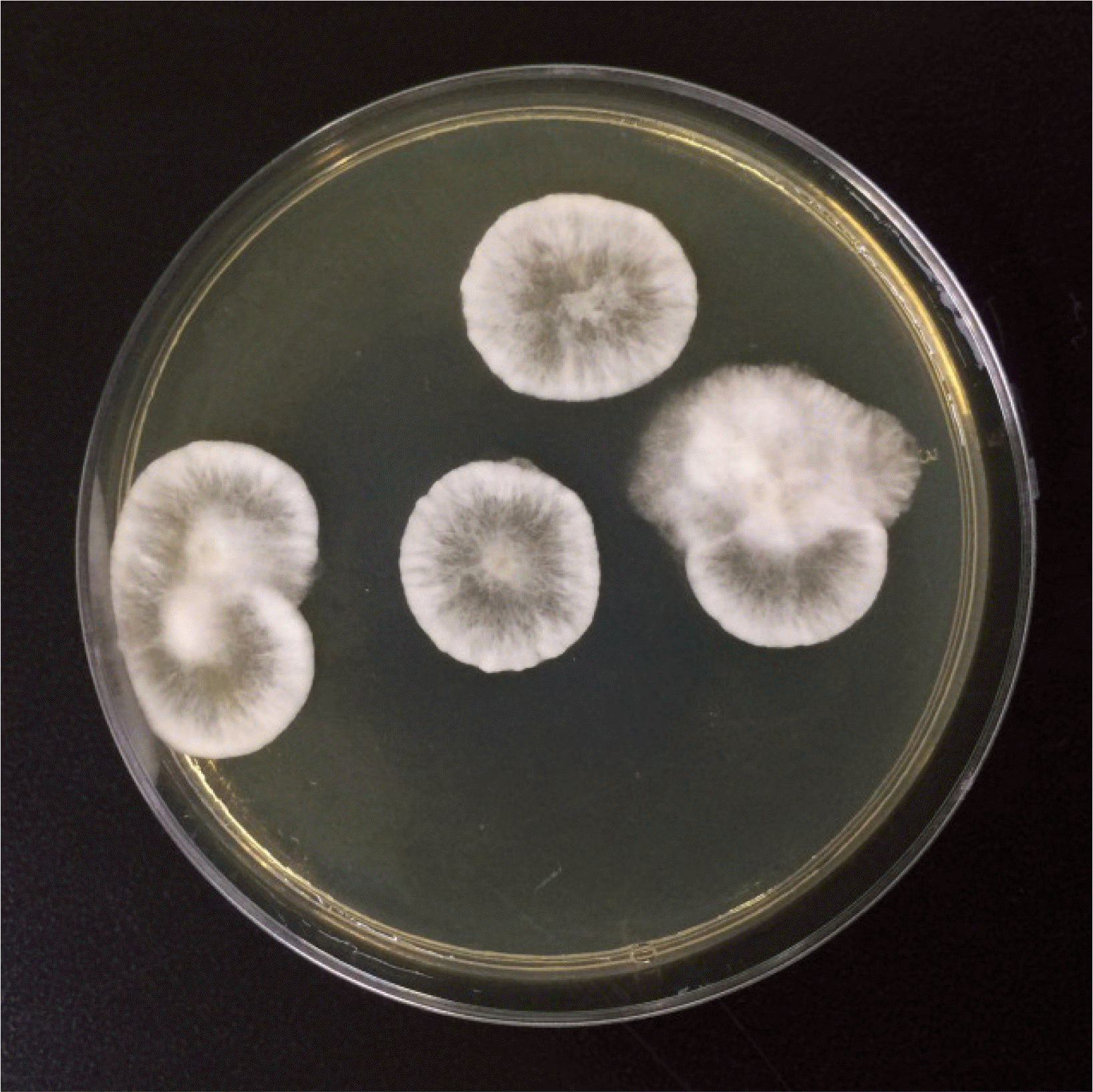
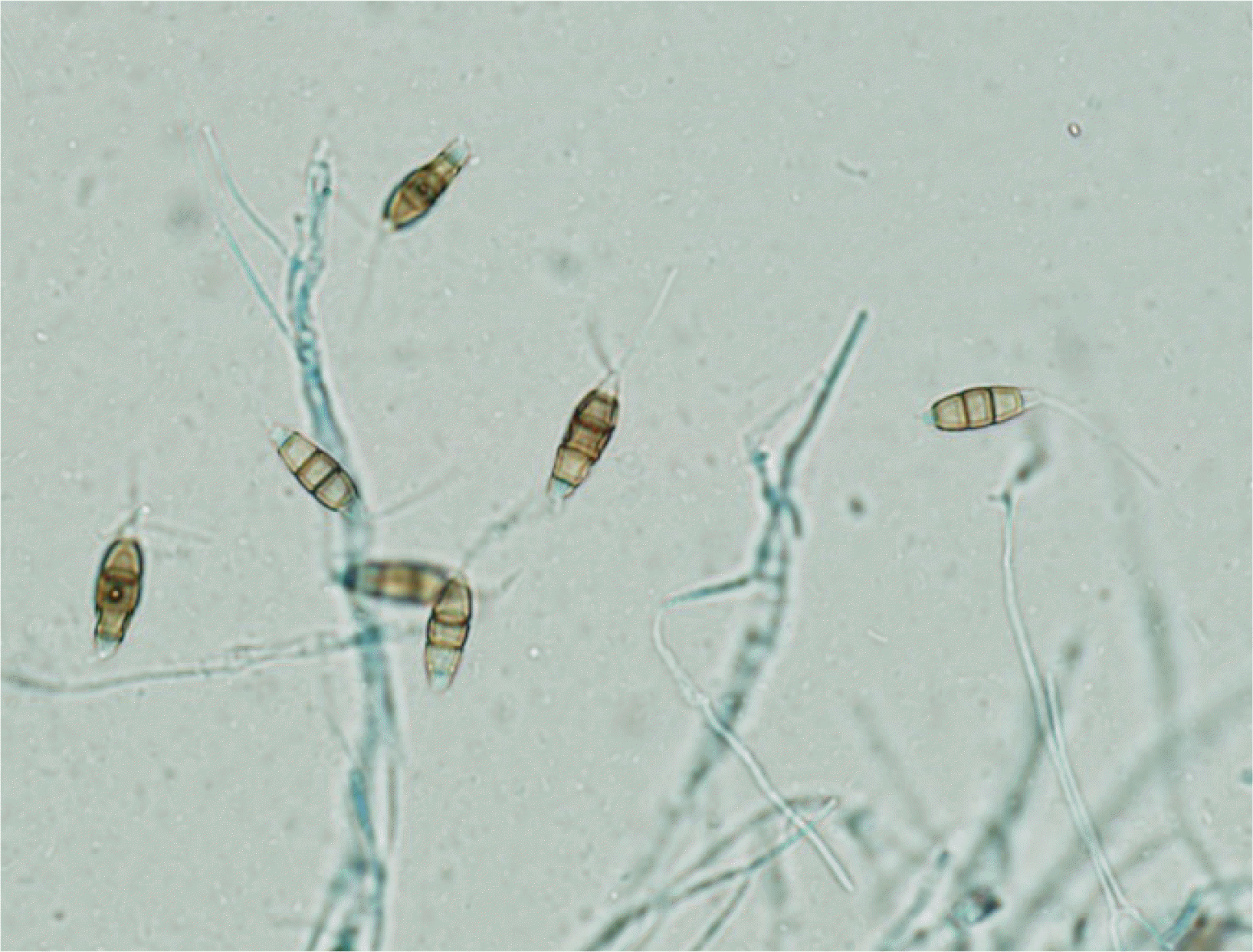
An 80-year-old male farmer was admitted following ocular pain in the right eye for two weeks. The patient had undergone surgery in both eyes for cataract and glaucoma two years prior to this incident. During initial presentation, the right conjunctiva was injected, and slit-lamp examination showed a 3.0×2.5 mm-sized epithelial defect in the cornea of the right eye, along with corneal thinning. Visual acuity measured via ¬nger counting was 30 cm in the right eye and 0.32 cm in the left eye. Intraocular pressure was not measured in the right eye, but was 9 mmHg in the left eye. KOH examination of corneal scrapings showed the presence of some spores; however, cultures for fungi showed negative results. Bacterial cultures of the corneal scrapings were also performed, and Serratia marcescens was identi¬ed. A treatment of moxi oxacin drops was initiated, and voriconazole was intravenously injected twice in a one-week interval. A month later, the second KOH examination and microbial culture were performed. The KOH examination revealed many hyphae, and a fungus was isolated from the fungal culture (Fig. 1 and 2). Species was identi¬ed using primers directed against an internal transcribed spacer region, and P. mangiferae was identi¬ed when the sequences corresponded 100% with GenBank Basic Local Alignment Search Tool database (GenBank accession no. JX305692.1).
Additional 200-mg voriconazole injections were administered twice in a two-week interval. Because the corneal perforation in the right eye did not resolve and visual acuity worsened, the patient was admitted for antifungal treatment and therapeutic penetrating keratoplasty (PKP). At the time of admission, a 3.2×3.2 mm-sized perforated and edematous cornea was observed in the right eye, and the anterior chamber of the right eye had nearly collapsed. Antibiotics (vancomycin, ceftazidime, and voriconazole) were applied to the anterior chamber, and intravitreal irrigation was performed during PKP surgery. Fungal and bacterial routine cultures performed on day 1 after surgery showed no growth. During follow-up in the outpatient clinic, no signs of infection were observed.
Go to : 
Although P. mangiferae is a plant pathogen that mainly affects M. indica, recent studies have discussed the extraction of novel anticancer, antibacterial, and antifungal compounds from P. mangiferae [1, 2]. Reports showing Pestalotiopsis as a causative agent of human or animal disease could not be found, except in one case of keratitis. Monden et al. were the ¬rst to report a case of fungal keratitis caused by P. clavispora [4].
Ophthalmic mycoses are being increasingly recognized as an important cause of morbidity and blindness, with keratitis (corneal infection) being the most frequent presentation. Fungi are opportunistic in terms of infecting the eye, since they rarely infect healthy, intact ocular tissues. Even the minor trauma of a dust particle falling onto the cornea can disrupt the integrity of the corneal epithelium, predisposing it to mycotic keratitis [5]. Common fungi that cause corneal infections include species of Fusarium, Aspergillus, Curvularia, Penicillium, and Candida. Although the evidence to guide treatment is limited, antifungal agents that are useful in treating fungal keratitis include topical natamycin and amphotericin B, oral or intravenous voriconazole, and intrastromal injection of voriconazole. Voriconazole has been reported to be particularly useful in treating cases of fungal keratitis using refractory and/or standard therapy such as topical natamycin or amphotericin B [6]. Marangon et al. [7] reported that common pathogens were susceptible to voriconazole in vitro at lowest minimum inhibitory concentrations (MICs) comparable to those of amphotericin B, uconazole, itraconazole, and ketoconazole.
Although antifungal agents such as natamycin, amphotericin B, and voriconazole are used to manage fungal keratitis, it is dif¬cult to treat rarely reported fungi due to delayed diagnosis or lack of evidence regarding the susceptibility of the infection to routinely used antifungal agents [8]. In their case, Monden et al. performed antifungal susceptibility tests for P. clavispora-induced fungal keratitis and found the MICs of most antifungal agents, including voriconazole, to be relatively high (2.0 μg/mL). Only micafungin demonstrated the lowest MIC (0.03 μg/mL). Thus, treatment with micafungin was initiated, and fungal in¬ltration of the cornea was resolved [4]. In the current case, approximately 3 months were required to culture and identify P. mangiferae as the pathogen. During this period, voriconazole treatment was continued; however, PKP surgery was performed before molecular identi-cation was completed because no response to the treatment was observed. Because the antifungal susceptibility tests for P. mangiferae were not conducted, it remains unknown whether the treatment failure was due to voriconazole resistance.
This is the ¬rst study to report a corneal P. mangiferae infection that not only failed to clear with voriconazole treatment, but also required PKP surgery. Thus, molecular identi¬cation and antifungal susceptibility tests may play an important role in determining patient treatment for cases where fungal keratitis is caused by a rare pathogen.
Go to : 
Acknowledgement
This research was supported by a fund (2019-ER5501-00) by Research of Korea Centers for Disease Control and Prevention.
Go to : 
REFERENCES
1. Kathiravan G, Sureban SM. 2009; Effect of taxol from Pestalotiopsis mangiferae on A549 cells-in vitro study. J Basic Clin Pharm. 1:1–9. PMID: 25206246. PMCID: PMC4158891.
2. Rai MK. 1996; In vitro evaluation of medicinal plant extracts against Pestalotiopsis mangiferae. Hindustan Antibiot Bull. 38:53–6. PMID: 9676046.
3. Subban K, Subramani R, Johnpaul M. 2013; A novel antibacterial and antifungal phenolic compound from the endophytic fungus Pestalotiopsis mangiferae. Nat Prod Res. 27:1445–9. DOI: 10.1080/14786419.2012.722091. PMID: 22950879.
4. Monden Y, Yamamoto S, Yamakawa R, Sunada A, Asari S, Makimura K, et al. 2013; First case of fungal keratitis caused by Pestalotiopsis clavispora. Clin Ophthalmol. 7:2261–4. DOI: 10.2147/OPTH.S48732. PMID: 24348013. PMCID: PMC3848927.
5. Thomas PA. 2003; Current perspectives on ophthalmic mycoses. Clin Microbiol Rev. 16:730–97. DOI: 10.1128/CMR.16.4.730-797.2003. PMID: 14557297. PMCID: PMC207127.

6. Austin A, Lietman T, Rose-Nussbaumer J. 2017; Update on the management of infectious keratitis. Ophthalmology. 124:1678–89. DOI: 10.1016/j.ophtha.2017.05.012. PMID: 28942073. PMCID: PMC5710829.

7. Marangon FB, Miller D, Giaconi JA, Alfonso EC. 2004; In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 137:820–5. DOI: 10.1016/j.ajo.2003.11.078. PMID: 15126145.

8. Maharana PK, Sharma N, Nagpal R, Jhanji V, Das S, Vajpayee RB. 2016; Recent advances in diagnosis and management of mycotic keratitis. Indian J Ophthalmol. 64:346–57. DOI: 10.4103/0301-4738.185592. PMID: 27380973. PMCID: PMC4966371.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download