This article has been
cited by other articles in ScienceCentral.
Abstract
Background
IgD is an immunoglobulin isotype that accounts for <1% of the total serum immunoglobulin, and IgD level changes can be observed in specific clinical situations. Herein, we evaluated the analytical performances of serum IgD quantification by the SPAPLUS turbidimetric analyzer (The Binding Site, UK), and determined a reference interval of IgD in the Korean population.
Methods
Precision, linearity and comparison with BNII (Siemens Healthineers, Germany) were assessed, and a reference interval was established according to the Clinical and Laboratory Standards Institute guidelines.
Results
Repeatability ranged from 0.78% to 2.14%, and the within-laboratory precision ranged from 4.10% to 5.40% for three concentrations. The coefficient of determination (R2) was 0.9996 for the analytical measurement range. The IgD concentration showed a correlation between SPAPLUS and BNII (y=0.890x-1.852, r=0.984). However, IgD concentrations tended to be lower in SPAPLUS compared to those in BNII at concentrations >100 mg/L. The established upper limit of the reference interval was 53.6 mg/L.
Conclusions
The SPAPLUS shows good analytical performance for serum IgD quantification.
Go to :

초록
배경
IgD는 혈청 총 면역글로불린의 1% 미만을 차지하는데, 특정한 임상 상황에서 농도 변화가 나타날 수 있다. 이 연구에서는 혼탁면역법을 이용하는 SPAPLUS 분석기(The Binding Site, UK)의 혈청 IgD 정량에 대한 성능 평가 및 한국인에서 IgD의 참고구간을 설정하였다.
방법
CLSI (Clinical and Laboratory Standards Institute) 가이드라인에 따라 정밀도와 직선성을 평가하였으며 참고구간 설정을 진행하였다. 또한 BNII (Siemens Healthineers, Germany) 장비와의 비교 분석을 함께 진행하였다.
결과
세 가지 농도 검체에 대하여 반복정밀도(%CV)는 0.78-2.14%, 검사실 내 정밀도(%CV)는 4.10-5.40%의 결과를 보였으며, 분석측정범위 내에서 직선성을 유지하였다(R2=0.9996). SPAPLUS와 BNII 장비 간 IgD 측정값은 다음과 같은 상관관계를 보였다(y=0.890x- 1.852, r=0.984). 하지만 100 mg/L 이상의 농도에서는 SPAPLUS가 BNII보다 낮은 농도를 보이는 경향이 있었다. 설정된 참고구간의 상한치는 53.6 mg/L이었다.
결론
SPAPLUS 장비는 혈청 IgD 정량에 대해서 우수한 검사 수행능을 보여주었다.
Go to :

Keywords: Analytical performance, IgD, Monoclonal gammopathy, Reference interval, Turbidimetry
INTRODUCTION
IgD, one of the immunoglobulin isotypes, was rst discovered in patients with multiple myeloma in 1965 [
1]. IgD accounts for <1% of the total serum immunoglobulin, and its clinical significance has not been completely established [
2]. Serum IgD concentration can be increased in some clinical situations, such as IgD monoclonal gammopathy, hyper-IgD syndrome, and autoimmune disease [
3,
4]. IgD multiple myeloma accounts for approximately 2% of multiple myeloma cases. Although IgD multiple myeloma is rare, early diagnosis is important because of its association with young age and advanced stage at the time of diagnosis [
5]. Particularly, IgD monoclonal peak is small or undetectable on electrophoresis, which emphasizes the importance of IgD quantification for diagnosis and disease monitoring [
6].
Serum IgD concentration shows a wide variation between normal individuals [
7,
8]. Previous studies have reported that several factors, such as age, sex, and smoking habit, can affect IgD concentration [
9-
11], although these findings are controversial [
12,
13].
Herein, we evaluated the analytical performance of the SPAPLUS (The Binding Site, Birmingham, UK) for IgD quantification and established a reference interval for IgD in the Korean population.
Go to :

MATERIALS AND METHODS
1. Reagents and instruments
The SPAPLUS was used according to the manufacturer’s instructions, using the SPAPLUS IgD Kit (product code: LK013.S, The Binding Site). The BNII system (Siemens Healthineers, Erlangen, Germany) was used according to the manufacturer’s instructions using IgD Latex Kit for use on the Siemens BNII (product code: LK013.T, The Binding Site).
At the standard 1/10 dilution, the measuring range is 7–210 mg/L. If the result was lower than the lower limit of the measuring range, the test was performed without dilution. If the result was higher than the upper limit of the measuring range, further dilution was performed to widen the measuring range to 16,800 mg/L. Therefore, the clinical reportable range is 0.7–16,800 mg/L.
2. Precision
Two control materials and one pooled serum were used for precision assessment. The estimated concentrations of control materials provided by the manufacturer were 23.1 mg/L and 97.7 mg/L. They were measured in duplicate per run, and two runs per day for 20 days. Repeatability and within-laboratory precision were evaluated according to the Clinical and Laboratory Standards Institute (CLSI) EP05-A3 guideline
14].
3. Linearity
Linearity was assessed according to the CLSI EP06-A guideline [
15] using the SPAPLUS calibrator, which consist of pooled human serum. The highest and lowest calibrators (0.6 mg/L and 20.8 mg/L, respectively) were mixed at ratios of 5:0, 4:1, 3:2, 2:3, 1:4, and 0:5. Each of the six concentrations was measured in duplicate. From these results, regression analysis was performed for the rst-, second-, and third-order polynomials, and the coef cient of determination (
R2) was calculated.
4. Comparison
Comparison with the BNII system was performed according to the CLSI EP09-A3 guideline [
16]. A total of 36 leftover patient samples evenly distributed at the analytical measurement range were included: 20 were within the reference interval claimed by the manufacturer, and 16 were outside the reference interval claimed by the manufacturer. The Wilcoxon signed-rank test, Passing-Bablok regression analysis, and Bland-Altman analysis were performed using MedCalc for Windows version 15.0 (MedCalc Software, Ostend, Belgium).
5. Reference interval
The reference interval was established according to the CLSI EP28-A3C guideline [
17] using MedCalc for Windows version 15.0 (MedCalc Software). A total of 120 samples from healthy individuals were included. The reference interval claimed by the manufacturer is 7.7–132.1 mg/L.
Go to :

RESULTS
1. Precision
The results of the precision evaluation are summarized in
Table 1. Repeatability ranged from 0.777% to 2.135% coef cient of variation (CV), and within-laboratory precisions ranged from 4.100% to 5.402% CV in three concentrations. Only within-laboratory precision of pooled serum exceeded 5% CV.
Table 1
Precision of the SPAPLUS in serum IgD quantification
|
Materials |
Mean (mg/L) |
SD |
CV (%) |
|
|
Repeatability |
Within-laboratory |
|
Low level QC |
23.7 |
0.939 |
1.089 |
4.100 |
|
High level QC |
101 |
4.224 |
0.777 |
4.289 |
|
Pooled serum |
15.9 |
0.812 |
2.135 |
5.402 |

2. Linearity
In the regression analysis, the rst-order equation was the best- tting line. Linearity was observed within the tested concentration range (0.6–20.8 mg/L) with a coef cient of determination (
R2) of 0.9996 (
Fig. 1).
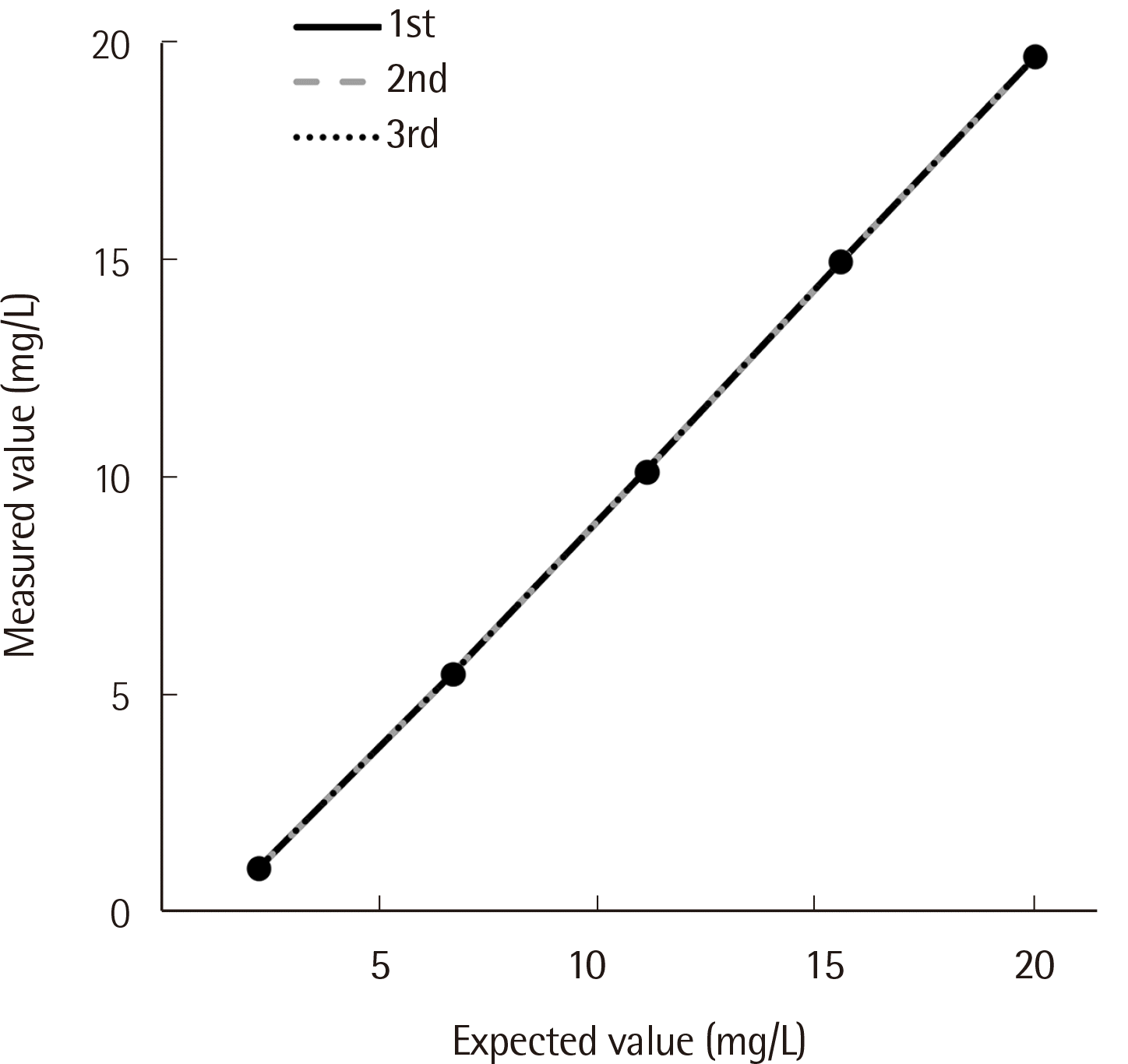 | Fig. 1Linearity of the SPAPLUS in serum IgD quantification. Linearity was observed within the tested concentration range (0.6–20.8 mg/L) with a coefficient of determination (R2) of 0.9996. 
|
3. Comparison
The IgD concentration showed a significant difference between the SPAPLUS and BNII (
P<0.0001) (
Fig. 2). The difference was not evident at concentrations <100 mg/L. In contrast, IgD concentrations tended to be lower in SPAPLUS than BNII at concentrations >100 mg/L. The correlation coefficient (
r) between SPA-PLUS and BNII was 0.984. The slope and intercept of the regression line were 0.890 (95% confidence interval, 0.790 to 0.966) and -1.852 (95% confidence interval, -3.167 to -0.647), respectively (
Fig. 3).
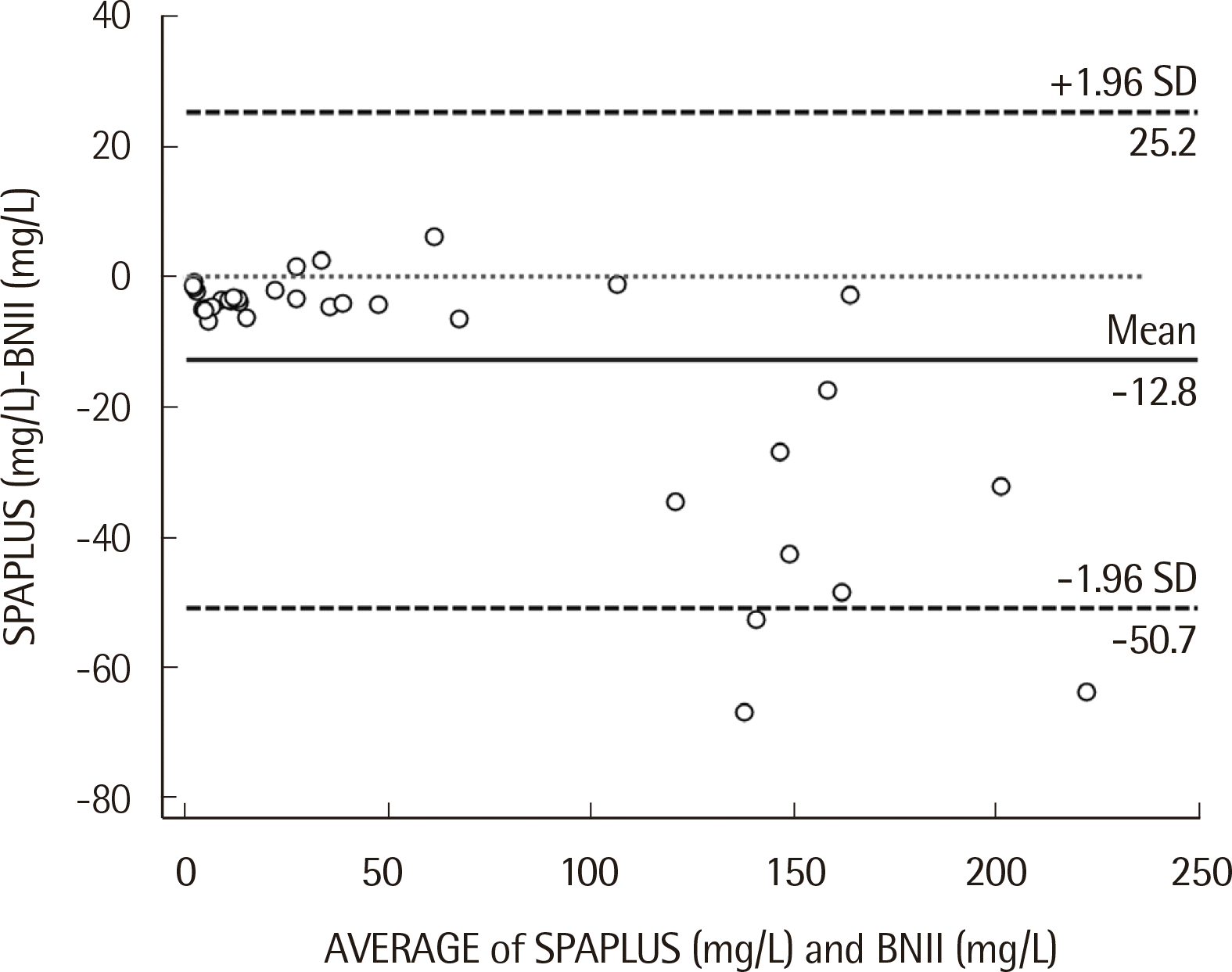 | Fig. 2Bland-Altman plots of serum IgD concentrations for comparison of the SPAPLUS and BNII. The IgD concentration showed a significant difference between the SPAPLUS and BNII (P<0.0001). The solid line is the mean difference, the dashed line is the limit of agreement, and the dotted line is the zero difference. 
|
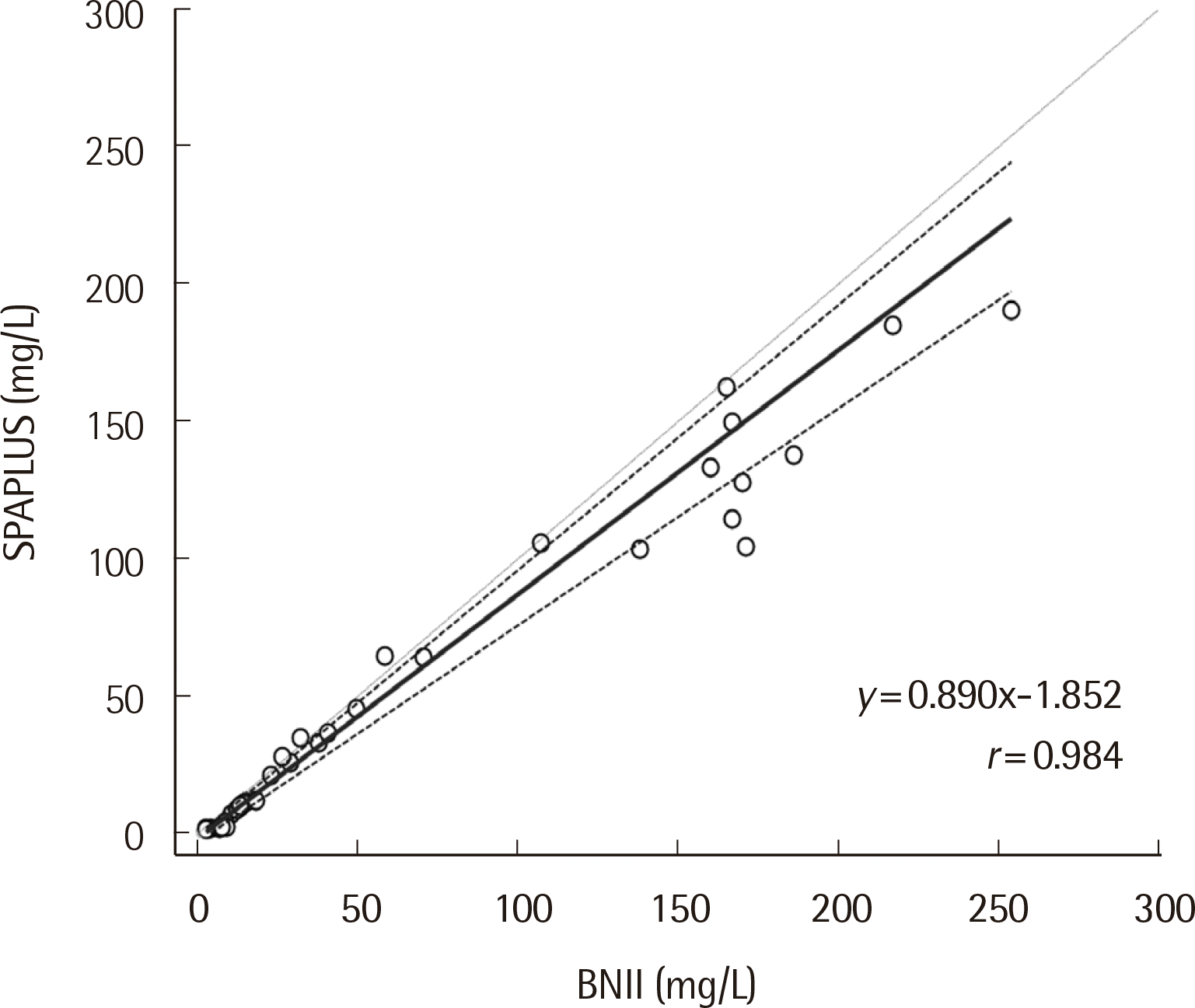 | Fig. 3Scatter diagram and Passing-Bablok regression line of serum IgD concentration for comparison of the SPAPLUS and BNII. The solid line is the regression line, the dotted line is the confidence interval, and the gray line is the identity line. 
|
4. Reference interval
The characteristics of the 120 healthy individuals are summarized in
Table 2. The histogram of IgD concentration from 120 samples showed a nonparametric distribution (
Fig. 4). Among the 120 samples, 65 were out of the reference interval claimed by the manufacturer; 64 were lower than the lower limit of the reference interval, and one was higher than the upper limit of the reference interval. Because the IgD distribution was skewed to the left and high concentrations are important for clinical decision-making, the right-sided 95% reference interval was calculated using nonparametric statistics. The established reference interval was <53.6 mg/L.
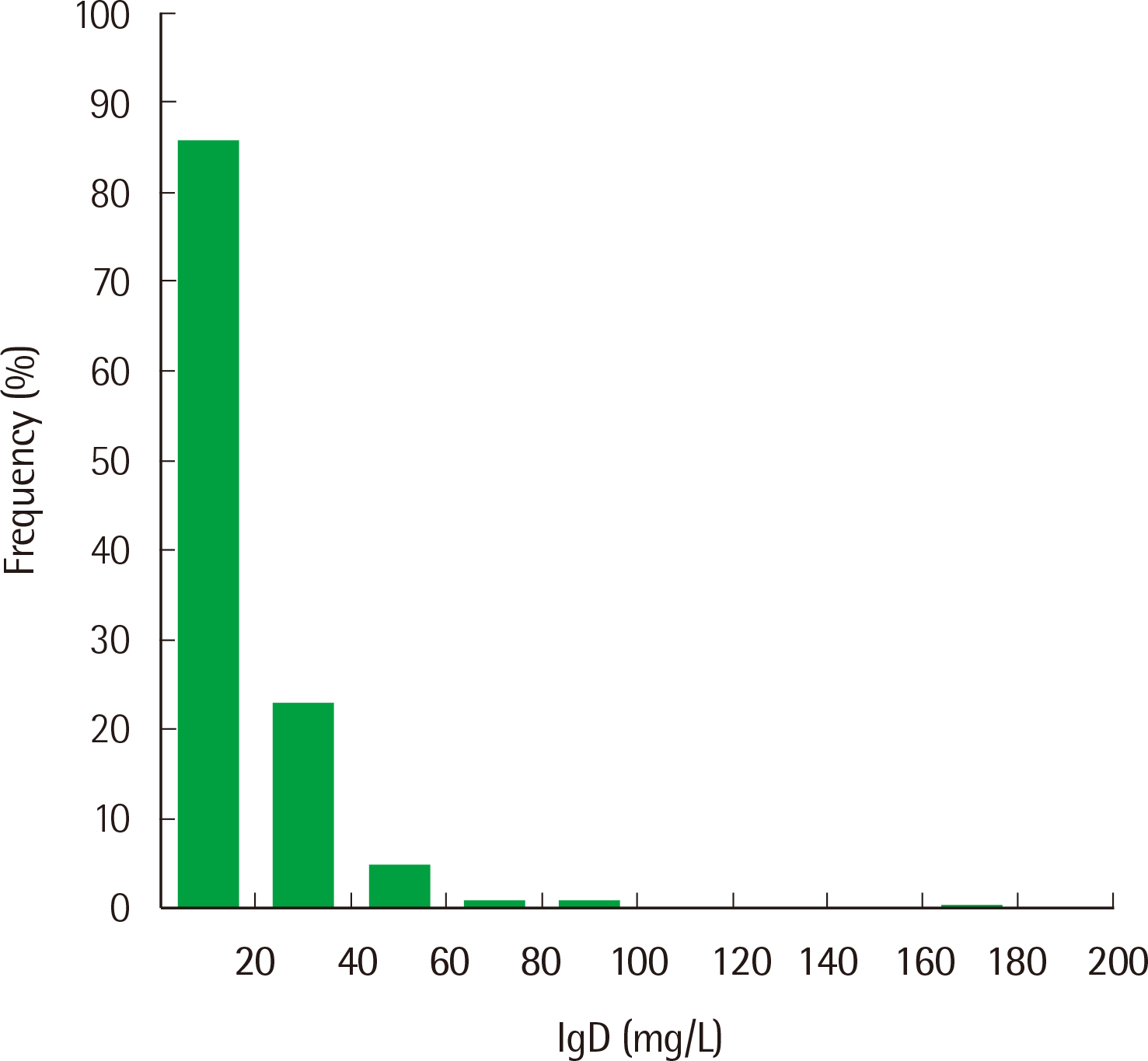 | Fig. 4Histogram of IgD concentration from 120 healthy individuals. 
|
Table 2
Characteristics of the 120 healthy individuals
|
Characterization |
Value |
|
Mean age (range), yr |
52 (20–78) |
|
Sex, N (%) |
|
|
Male |
47 (47.5) |
|
Female |
63 (52.5) |
|
Smoking history, N (%) |
|
|
Current smoker |
17 (14.2) |
|
Ex-smoker |
19 (15.8) |
|
Never smoker |
52 (43.3) |
|
Not evaluated |
32 (26.7) |

Go to :

DISCUSSION
IgD is considered a relatively non-significant immunoglobulin isotype. Although previous studies have revealed that IgD is related to several clinical situations, such as IgD monoclonal gammopathy, hyper-IgD syndrome, and autoimmune disease, the clinical signi cance of IgD is still unknown [
2]. However, IgD quantification is important in the diagnosis and monitoring of IgD monoclonal gammopathy [
6]. Therefore, we evaluated the analytical performance of the SPAPLUS turbidimetric analyzer for IgD quantification.
Our study demonstrated good analytical performance of the SPAPLUS turbidimetric analyzer for IgD quantification. The evaluation of precision, linearity, and comparison between the SPAPLUS and BNII showed satisfactory results. Although SPAPLUS showed good correlation with BNII (r=0.984), IgD concentration showed a significant difference between the SPAPLUS and BNII (P<0.0001). The difference was evident at concentrations >100 mg/L on the Bland-Altman plot. At this range, IgD concentration tended to be lower in the SPAPLUS than in the BNII, which could affect patient assessment, especially in IgD monoclonal gammopathy. The stability issue of high concentration samples might be possible cause of the difference between two instruments. Because it was extremely difficult to collect a sufficient number of high concentration samples for comparison, we used high concentration samples kept by other institutions, which might affect the stability of the samples.
The reference interval established in this study (0–53.6 mg/L) was narrower than that claimed by the manufacturer (reagents LK013.S and LK013.T, 7.7–132.1 mg/L and 1.3–152.7 mg/L, respectively). The difference between reference intervals claimed by the manufacturer (reagents LK013.S and LK013.T) and those established in this study might be due to differences in several factors in individuals included in each study. Because smoking is a well-known factor affecting IgD concentration [
9-
11], we retrospectively reviewed the smoking history of 120 individuals included in the reference interval establishment in this study. Of 120 individuals, 32 had no information on smoking history. The remaining 88 individuals consisted of 59.1% (52/88) never smokers, 19.3% (17/88) current smokers, and 21.6% (19/88) ex-smokers. Applying the reference interval claimed by the manufacturer (LK013.S), 64 individuals had concentrations not within the lower limit of reference interval: 29 never smokers, 9 current smokers, 8 ex-smokers, and 18 individuals without information on smoking history. Except for the 64 individuals, median IgD concentrations were higher in ex-smokers and current smokers than in never smokers (30.5 mg/L, 30.4 mg/L, and 22.0 mg/L, respectively). This nding was consistent with previous studies that indicated positive association between IgD concentration and smoking [
9-
11]. However, unfortunately, because the package insert provided by the manufacturer did not include smoking information for individuals included in the reference interval establishment, we could not conclude that smoking was the contributing factor to the difference in reference intervals. Some previous studies reported the in uence of sex on IgD concentrations: IgD concentrations were higher in men [
10,
12]. However, some studies show no significant difference in IgD concentrations between men and women [
2,
9]. Therefore, we additionally compared the IgD concentrations in men and women using 120 healthy individuals, but there was no significant difference (
P=0.0746).
Ethnicity was another possible factor causing a difference in reference intervals. The reference intervals claimed by the manufacturer (reagents LK013.S and LK013.T) were based on the US and UK populations, respectively. In contrast, this study was conducted in the Korean population. Although a previous study of Gerrard et al. showed different IgD concentrations in different ethnic populations [
18], there have been few studies that have addressed the association of IgD concentration and ethnicity. Further studies are needed to establish an association between IgD concentration and ethnicity.
In conclusion, the SPAPLUS turbidimetric analyzer shows good analytical performance for IgD quantification. At a high concentration, results from the SPAPLUS and BNII showed a significant difference. In addition, because the reference interval for IgD can be affected by several factors, it is recommended to consider these factors when establishing a reference interval.
Go to :






 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download