This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The platelet aggregation test is widely used to measure antiplatelet therapy response and to detect platelet function disorders. CS-5100 (Sysmex Co., Japan) is a recently introduced coagulation analyzer that can also measure platelet aggregation. We evaluated the performance of CS-5100 in the platelet aggregation test for use in clinical laboratories.
Methods
We investigated the precision, stability, dilution test, and correlation of CS-5100 with a traditional aggregometer. Precision was tested using normal and patient samples. Stability was assessed over 5 different time points for 8 hours. The dilution test was performed with normal samples using ADP agonists. We tested correlations between the results of Chrono-log aggregometer (Chrono-Log Co., USA) and CS-5100 using 10 samples with 5 agonists each. We also calculated the reference range of 5 agonists using 22-30 normal samples.
Results
The coefficients of variation using normal samples were 7.45% and 3.27% for ADP and arachidonic acid, respectively. Stability was maintained for up to 2 hours in most samples. Dilution tests showed similar results until reaching a dilution factor of 2. Strong correlations of the results between Chrono-log and CS-5100 were found, except for ristocetin. The reference ranges of 5 reagents in CS-5100 were similar to those obtained with the Chrono-log aggregometer.
Conclusions
The performance of CS-5100 in platelet aggregation tests showed reliable results compared to a traditional aggregometer. CS-5100 can perform coagulation test and platelet aggregation test, simultaneously. Thus, CS-5100 can enable cost saving and reduce turn-around-time by reducing the inspection time.
Go to :

초록
배경
혈소판 응집검사는 항 혈소판치료 반응을 평가하고 혈소판 기능장애를 진단하는 데 널리 사용되는 검사이다. CS-5100 (Sysmex Co., Japan)은 최근 소개된 응고기능검사 장비로 혈소판 응집검사도 시행 가능하다. 저자들은 검사실에서의 CS-5100의 혈소판 응집검사의 성능을 평가하였다.
방법
저자들은 CS-5100의 정밀도, 안정성, 희석검사, 그리고 전통적인 장비와의 상관성을 평가하였다. 정밀도는 정상 및 환자 검체로 시행하였다. 안정성은 8시간 동안 5번 측정하여 평가하였다. 희석검사는 ADP시약을 사용하여 정상 검체를 이용하여 평가하였다. 또한 전통적인 응집검사 장비와의 상관성을 평가하기 위해 Chrono-log사의 응집검사 장비(Chrono-Log Co., USA)와 5개의 시약을 각각 10개의 검체를 이용하여 비교하였다. 저자들은 또한 22-30개의 정상 검체를 이용하여 5가지 시약의 참고범위도 측정하였다.
결과
정상 검체에서의 변이계수는 ADP시약에서는 7.45%, Arachidonic acid 시약에서는 3.27%였다. 안정성은 대부분의 검체에서 2시간까지 유지되었다. 희석검사 또한 1/2 희석배수까지 비슷한 결과를 보였다. Ristocetin시약을 제외한 나머지 시약에서는 모두 Chrono-log 장비와 강한 상관성을 나타내었다. CS-5100에서의 5가지 시약의 참고범위는 Chrono-log사의 값과 비슷하였다.
결론
전통적인 장비와 비교하였을 때, CS-5100은 혈소판 응집검사에서 신뢰할 만한 결과를 보였다. CS-5100은 응고검사와 혈소판 응집검사를 동시에 수행할 수 있는 장비이다. 그러므로, CS-5100의 사용은 검사비용을 줄이고 검사 소요시간을 단축시킬 수 있을 것이다.
Go to :

Keywords: CS-5100, Platelet aggregation test, Coagulation instrument, ADP, Arachidonic acid
INTRODUCTION
Platelets are blood cells present in peripheral blood. They are responsible for primary hemostasis through adhesion and coagulation [
1]. They are mainly produced in the bone marrow and are associated with various diseases such as immune thrombocytopenic purpura, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, essential thrombocythemia, Bernard-Soulier Syndrome, and Glanzmann’s thrombasthenia due to numerical abnormalities and dysfunction [
2,
3]. Various tests are performed in hospitals to measure platelet function. Among these, the platelet aggregation test is widely used to measure antiplatelet therapy responses and to detect congenital and acquired platelet function disorders [
4,
5]. Light transmission aggregometry is the most common platelet aggregation test [
6]. Conventional analyzers are usually semi-automated with result analysis and aggregation detection performed automatically, whereas sample dispensing and ag-onist addition performed manually.
The CS-5100 (Sysmex Co., Kobe, Japan) is an automated blood coagulation analyzer that can also measure platelet aggregation. The CS-5100 is a fully automated analyzer that is convenient and saves time. It uses several agonists for the platelet aggregation test, including ADP, arachidonic acid, collagen, epinephrine, and ristocetin [
7]. Blood sampling and centrifugation are performed manually in CS-5100; however, platelet rich plasma (PRP) and platelet poor plasma (PPP) are dispensed automatically, and agonist addition is also automatic. We evaluated the performance of CS-5100 in evaluating platelet aggregation for use in clinical laboratories. We measured the precision, stability, dilution test, correlation of the two instruments, and the reference range of five reagents.
Go to :

MATERIALS AND METHODS
1. Instruments
The CS-5100 is used in our laboratory for blood coagulation tests. It also can be used to measure platelet aggregation. It performs the platelet aggregation tests using light transmission aggregometry. After preparing the sample and reagent, the instrument analyzes the PRP and PPP samples and then automatically calculates the aggregation %. Aggregation % is calculated using PRP results, PPP results, and the degree of absorbance. The range of the results is from -25% to 100%. A Chrono-log aggregometer (Chrono-Log Co., Havertown, PA, USA) was used for the correlation test.
2. Samples
This study was approved by the institutional review board of Dong-A University Hospital (Busan, Korea). The evaluated blood samples were collected in 3.2% sodium citrate tubes (Greiner Bio-One, Frickenhausen, Germany) and mixed gently. To obtain PRP samples, samples were centrifuged at 200 g for 10 minutes at room temperature. The resultant PRP samples were transferred to a plastic tube using a plastic pipette. To obtain PPP samples, samples were centrifuged at 1,500 g for 15 minutes at room temperature. The resultant PPP samples were also transferred to a plastic tube. Platelet counts in PPP samples were less than 10×109/L. Normal samples were obtained from a health screening center, and patient samples were residual samples obtained from patients with cerebrovascular disease such as cerebral infarction, cerebral hemorrhage, and so on.
3. Reagents
CHRONO-PAR Reagents (BioTop Medical, Leiden, Netherlands) were used for the platelet aggregation tests and included ADP, arachidonic acid, collagen, epinephrine, ristocetin, and thrombin. Among these, five reagents were used for platelet aggregation tests, including ADP, arachidonic acid, collagen, epinephrine and ristocetin. The final concentrations of the reagents were determined according to the guidelines of the International Society of Thrombosis and Hemostasis (ISTH) [
8]. The concentrations of ADP, arachidonic acid, collagen, epinephrine, and ristocetin were 2 μM, 1.0 mM, 2 μg/mL, 5 μM, and 1.2 mg/mL, respectively.
4. Precision
Normal and patient samples were also tested to evaluate precision. The test was repeated five times within one day. The reagents used were ADP and arachidonic acid. Mean values, SD and CVs were calculated. The test was performed following the Clinical and Laboratory Standard Institute (CLSI) guidelines EP5-A3 [
9].
5. Stability
Stability was assessed by measuring normal and patient samples at five different times. The test was performed immediately and at 1 hour, 2 hours, 4 hours, and 8 hours after phlebotomy using ADP and arachidonic acid. The results of stability were analyzed statistically by Mann-Whitney test.
6. Dilution tests
Dilution tests were performed to measure the effect of platelet counts. Dilution tests were only performed with normal samples. The tests were performed with several dilution factors of 1, 2, 4, 8, 16, 32, 64, and 128. The agonists ADP and arachidonic acid were used to analyze the dilution tests. We also measured the platelet counts.
7. Correlation tests
Correlation tests were performed following the CLSI guidelines EP9-A3 [
10]. Correlations between the results of Chrono-log aggregometer and CS-5100 were assessed using 10 samples with five reagents (ADP, arachidonic acid, collagen, epinephrine, ristocetin) each. The concentrations of the reagents used in CS-5100 were determined using ISTH guidelines [
8]. Correlation between the CS-5100 and Chrono-log aggregometer was analyzed using Microsoft Excel 2010 (Microsoft Co., Redmond, WA, USA).
8. Reference ranges
The reference ranges of the five reagents were investigated using 22-30 normal samples based on the calculated mean±2SD of all test samples. Tests to measure reference ranges were performed following the CLSI guidelines EP28-A3C [
11]. The numbers of samples used for the assay were 27, 22, 30, 30, and 30 for ADP, arachidonic acid, collagen, epinephrine, and ristocetin, respectively.
9. Statistical analyses
All statistical analyses were performed with Microsoft Excel 2010 (Microsoft Co.) and Med-Calc software v16.4.3 for Windows (Med-Calc, Ostend, Belgium). A P-value less than 0.05 was regarded as statistically significant.
Go to :

RESULTS
1. Precision
The results of precision testing are shown in
Table 1. The CVs using normal samples were 7.45% and 3.27% for ADP and arachidonic acid, respectively. However, the CVs using patient samples were 27.22% and 121.35% for ADP and arachidonic acid, respectively.
Table 1
Within day precision of CS-5100 platelet aggregation analysis
|
Parameter |
Normal (aggregation %) |
Abnormal (aggregation %) |
|
ADP |
|
1 |
69.7 |
17.8 |
|
2 |
81.4 |
16.4 |
|
3 |
77.2 |
20.4 |
|
4 |
85.5 |
29.0 |
|
5 |
79.4 |
29.2 |
|
Mean |
78.64 |
22.56 |
|
SD |
5.86 |
6.14 |
|
CV |
7.45 |
27.22 |
|
Arachidonic acid |
|
1 |
94.2 |
6.8 |
|
2 |
91.9 |
2.4 |
|
3 |
92.3 |
-0.4 |
|
4 |
87.7 |
0.4 |
|
5 |
95.7 |
2.3 |
|
Mean |
92.36 |
2.3 |
|
SD |
3.02 |
2.79 |
|
CV |
3.27 |
121.35 |

2. Stability
The results of stability testing are shown in
Table 2. In normal ADP samples, stability at 4 hours after phlebotomy was signifi-cantly low (92.7% (88.9–97.6) vs. 63.3% (40.9–86.6);
P =0.0495). In normal arachidonic acid samples, stability at 8 h after phlebotomy was significantly low (85.9% (85.7–93.7) vs. 3.4% (1.9–77.8);
P =0.0495). In patient ADP samples, stability at 8 hours after phlebotomy was low, but this result was not statistically significant (67.7% (42.0–71.5) vs. 26.3% (-23.0–58.3);
P =0.1266). In patient arachidonic acid samples, the stability results were too low to be analyzed.
Table 2
Stability test of CS-5100 platelet aggregation assay
|
Groups |
Agonist |
Sample No. |
0 hr |
1 hr |
2 hr |
4 hr |
8 hr |
|
Normal |
ADP |
1 |
97.6 |
85.6 |
89.0 |
63.3 |
35.6 |
|
2 |
92.7 |
73.5 |
73.6 |
40.9 |
28.8 |
|
3 |
88.9 |
91.4 |
92.5 |
86.6 |
63.9 |
|
Mean |
93.1 |
83.5 |
85.0*
|
63.6 |
42.8 |
|
Arachidonic acid |
1 |
85.7 |
94.2 |
91.9 |
95.5 |
1.9 |
|
2 |
85.9 |
83.8 |
82.7 |
85.6 |
3.4 |
|
3 |
93.7 |
90.2 |
89.6 |
83.6 |
77.8 |
|
Mean |
88.4 |
89.4 |
88.1 |
88.2*
|
27.7 |
|
Patient |
ADP |
1 |
71.5 |
76.4 |
67.7 |
68.0 |
58.3 |
|
2 |
67.7 |
69.1 |
65.5 |
60.6 |
-23.0 |
|
3 |
42.0 |
48.4 |
47.2 |
39.0 |
26.3 |
|
Mean |
60.4 |
64.6 |
60.1 |
55.9*
|
20.5 |
|
Arachidonic acid |
1 |
1.4 |
0.5 |
2.3 |
2.4 |
1.7 |
|
2 |
2.3 |
1.7 |
1.2 |
1.6 |
-0.3 |
|
3 |
8.1 |
1.5 |
-0.5 |
1.6 |
1.8 |
|
Mean |
3.9 |
1.2 |
1.0 |
1.9 |
1.1 |

3. Dilution tests
Dilution test results are also displayed in
Table 3. The initial platelet count of the normal samples was 377,000/μL. Tests using ADP and arachidonic samples showed similar results until reaching a dilution factor of 2.
Table 3
Dilution test using normal ADP samples
|
Sample No. |
Dilution factor |
Platelet count (/μL) |
ADP (aggregation %) |
Arachidonic acid (aggregation %) |
|
1 |
1 |
377,000 |
85.6 |
90.0 |
|
2 |
1/2 |
193,000 |
89.9 |
91.4 |
|
3 |
1/4 |
98,000 |
85.6 |
7.2 |
|
4 |
1/8 |
51,000 |
6.9 |
2.5 |
|
5 |
1/16 |
24,000 |
-15.1 |
-3.1 |
|
6 |
1/32 |
12,000 |
-8.3 |
5.6 |
|
7 |
1/64 |
6,000 |
-3.8 |
0.0 |
|
8 |
1/128 |
3,000 |
-3.1 |
0.0 |

4. Correlation with a traditional aggregometer
The correlation test results are displayed in
Fig. 1. In ADP and arachidonic acid treatment samples, the results of the CS-5100 and Chrono-log aggregometer were strongly correlated (R=0.955, R= 0.996, respectively) [
12,
13]. The results of collagen and epinephrine samples were correlated, but not very strongly (R=0.881, R= 0.895, respectively) [
12,
13]. Finally, ristocetin addition revealed a modest correlation result (R=0.586) [
12,
13].
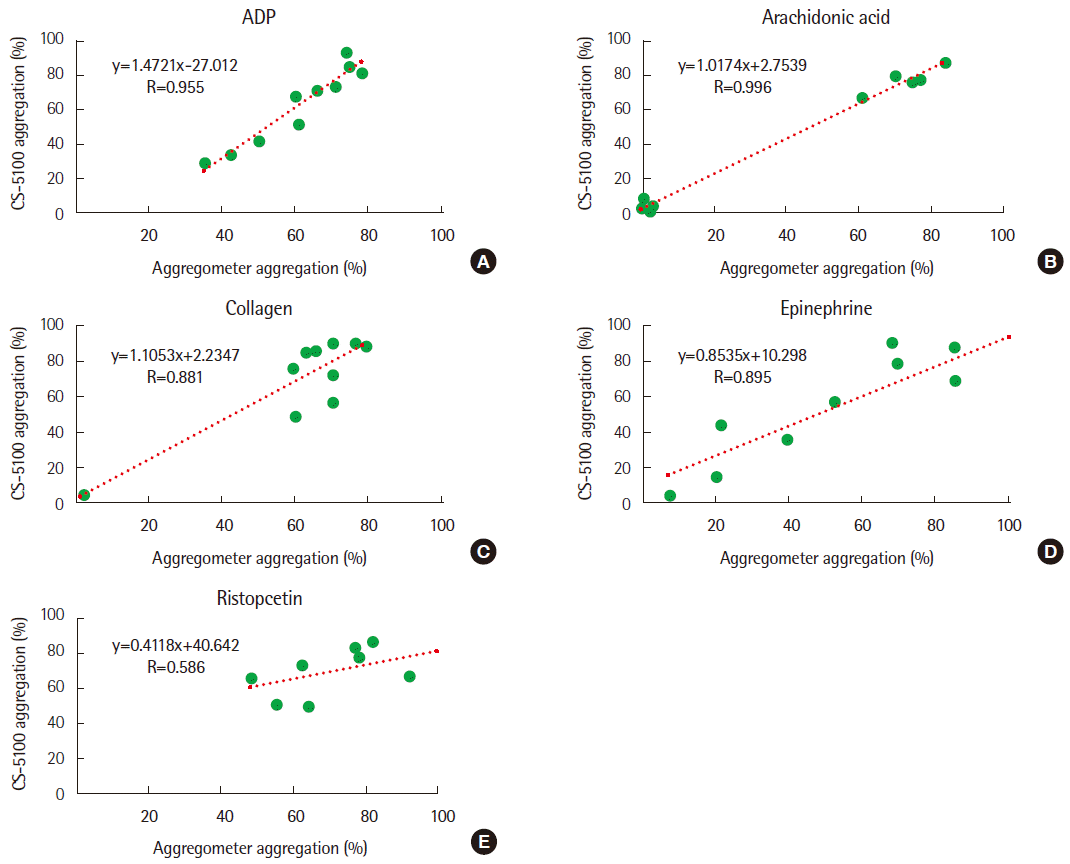 | Fig. 1Correlation of the test results between Chrono-log aggregometer and CS-5100 aggregation assay. (A) ADP, (B) Arachidonic acid, (C) Collagen, (D) Epinephrine, and (E) Ristocetin. 
|
5. Reference ranges of the CS-5100 platelet aggregation test
The test results are shown in
Table 4. All tests using five agonists showed normal distribution; the reference range was calculated as mean±2SD. The reference ranges were 59.3-105.2% for ADP, 63.2-102.0% for arachidonic acid, 74.0-102.7% for collagen, 72.8-104.3% for epinephrine, and 69.9-97.8% for ristocetin.
Table 4
Reference range of CS-5100 automated platelet aggregation assay
|
Agonist |
N |
Mean (%) |
SD |
Mean±2SD (%) |
|
ADP |
27 |
82.3 |
11.5 |
59.3-105.2 |
|
Arachidonic acid |
22 |
82.6 |
9.7 |
63.2-102.0 |
|
Collagen |
30 |
88.3 |
7.2 |
74.0-102.7 |
|
Epinephrine |
30 |
88.6 |
7.9 |
72.8-104.3 |
|
Ristocetin |
30 |
83.8 |
7.0 |
69.9-97.8 |

Go to :

DISCUSSION
Platelet aggregation tests are being increasingly used because of their usefulness in diagnosis and to monitor therapeutic response. The fully automated CS-5100 coagulation and platelet aggregation analyzer is thus expected to be more useful in hospitals and laboratories.
The CS-5100 analyzer yielded acceptable precision with normal samples using both ADP and arachidonic acid. However, the results of patient samples were not acceptable. The number of sam-ples was only five and the aggregation test results were too low; thus, the CV results of patient samples were very high and not acceptable. Therefore, more samples and additional reagents are required for further performance evaluation.
The CS-5100 provided good stability in both normal and patient samples using ADP and arachidonic acid. Results of ADP in normal samples were adequate until 2 hours after phlebotomy. Results using arachidonic acid in normal samples were acceptable until 4 hours after phlebotomy. In patient samples, results using ADP in samples were good until 4 hours after phlebotomy. Results using arachidonic acid in patient samples were all similar after phlebotomy, but were very poor and could not be analyzed. Thus, more samples and additional evaluation are required for accurate performance measurements. These results indicate that when using CS-5100, tests should be performed within 2 hours after phlebotomy.
Tests using ADP and arachidonic acid are most frequently used in the clinical laboratory, and so, dilution tests were performed with ADP and arachidonic acid agonists. In dilution tests, the re-sults using ADP and arachidonic samples were adequate until 2× dilution. Thus, if the platelet counts of samples are more than 200,000/μL, the results of aggregation were reliable.
In a comparison test between the CS-5100 and Chrono-log aggregometer, the results using four reagents except ristocetin showed strong correlations. Use of ADP and arachidonic acid showed strong correlations (R=0.955, R=0.996, respectively) [
12,
13]. Use of collagen and epinephrine showed relatively weaker correlation (R= 0.881, R=0.895, respectively) [
12,
13]. In contrast, the results of ris-tocetin showed a modest correlation (R=0.586) [
12,
13]. These results are likely caused because of not using a dedicated reagent or using the wrong concentration of the reagent obtained from the manufacturer.
The reference ranges of CS-5100 were not significantly different from those of traditional aggregometers [
14,
15]. In reference range tests, using ADP and arachidonic acid provide wider ranges than with the other three reagents. However, the numbers of samples used for the assay was small; thus, additional samples are required for further performance evaluation.
The results of the CS-5100 platelet aggregation test are similar to other results reported using CS-2100i [
16,
17]. However, our analysis has some limitations. First, because of a small number of samples, the SD and CV results were not good. We only tested ADP and arachidonic acid in precision, stability, and dilution tests. The precision tests were performed only five times, and so, their results might not be reliable. Second, the reagents were not dedicated for CS-5100, and were also used for the Chrono-log aggregometer. Therefore, the results of some tests were not acceptable. Further, additional samples are needed to evaluate accuracy. Dedicated reagents for CS-5100 are needed in further evaluations. Third, only samples from patients with some cerebrovascular diseases were used; thus, additional tests are needed with samples of patients with coagulation disorders such as Glanzmann’s thrombasthenia, Bernard-Soulier Syndrome, and so on.
Despite some limitations, the performance of the CS-5100 platelet aggregation test was reliable in terms of precision, stability, and dilution tests. The CS-5100 showed results comparable to those of a traditional aggregometer and has many advantages as it can execute coagulation tests and platelet aggregation tests without an additional aggregometer. It can also reduce the complexity of former aggregometers and manual tests. Therefore, we expect that the use of CS-5100 will be profitable in many ways to save time and increase convenience.
Go to :

Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2018- R1A1A3A04078765).
Go to :

Notes
Go to :

REFERENCES
2. Nurden AT, Nurden P. 2014; Congenital platelet disorders and understanding of platelet function. Br J Haematol. 165:165–78. DOI:
10.1111/bjh.12662. PMID:
24286193.

6. Cattaneo M, Hayward CP, Moffat KA, Pugliano MT, Liu Y, Michelson AD. 2009; Results of a worldwide survey on the assessment of platelet function by light transmission aggregometry: a report from the platelet physiology subcommittee of the SSC of the ISTH. J Thromb Haemost. 7:1029. DOI:
10.1111/j.1538-7836.2009.03458.x. PMID:
19422455.

7. Sakayori T, Watanabe Y, Nakajima K, Misawa E, Kobayashi K, Kurono H, et al. 2016; Introduction and evaluation of light transmission platelet aggregation method on the Sysmex CS-series automated coagulation analyzer. Sysmex Journal International. 26:1–10.
8. Cattaneo M, Cerletti C, Harrison P, Hayward CP, Kenny D, Nugent D, et al. 2013; Recommendations for the standardization of light transmission aggregometry: a consensus of the working party from the platelet physiology subcommittee of SSC/ISTH. J Thromb Haemost. 11:1183–9. DOI:
10.1111/jth.12231. PMID:
23574625.

9. Clinical and Laboratory Standard Institute. 2014; Evaluation of precision of quantitative measurement procedures; Approved guideline-third edition. CLSI document EP05-A3. Clinical and LaboratoryStandard Institute;Wayne, PA:
10. Clinical and Laboratory Standard Institute. 2013; Measurement procedure comparison and bias estimation using patient samples; Approved guideline-third edition. CLSI document EP09-A3. Clinical and LaboratoryStandard Institute;Wayne, PA:
11. Clinical and Laboratory Standard Institute. 2010; Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved guideline-third edition. CLSI document EP28-A3C. Clinical and LaboratoryStandard Institute;Wayne, PA:
12. Taylor R. 1990; Interpretation of the correlation coefficient: a basic review. J Diagn Med Sonogr. 6:35–9. DOI:
10.1177/875647939000600106.

13. Mukaka MM. 2012; Statistics corner: A guide to appropriate use of correla-tion coefficient in medical research. Malawi Med J. 24:69–71. PMID:
23638278. PMCID:
PMC3576830.
14. Hayward CP, Moffat KA, Pai M, Liu Y, Seecharan J, McKay H, et al. 2008; An evaluation of methods for determining reference intervals for light transmission platelet aggregation tests on samples with normal or reduced platelet counts. Thromb Haemost. 100:134–45. DOI:
10.1160/TH08-03-0183.

15. Miller CH, Rice AS, Garrett K, Stein SF. 2014; Gender, race and diet affect platelet function tests in normal subjects, contributing to a high rate of abnormal results. Br J Haematol. 165:842–53. DOI:
10.1111/bjh.12827. PMID:
24617520. PMCID:
PMC4477706.

16. Ling LQ, Liao J, Niu Q, Wang X, Jia J, Zuo CH, et al. 2017; Evaluation of an automated light transmission aggregometry. Platelets. 28:712–9. DOI:
10.1080/09537104.2016.1265923. PMID:
28150526.

17. Lawrie AS, Kobayashi K, Lane PJ, Mackie IJ, Machin SJ. 2014; The automation of routine light transmission platelet aggregation. Int J Lab Hema-tol. 36:431–8. DOI:
10.1111/ijlh.12161. PMID:
24237750. PMCID:
PMC4298021.

Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download