2. Son KB, Shin JW, Lim EO, Lee TJ, Kim HS. Population aging and health care expenditure in South Korea: a critical review. Korean J Health Econ Policy. 2015; 21:51–77.
3. Zeng Y, Gu D, Purser J, Hoenig H, Christakis N. Associations of environmental factors with elderly health and mortality in China. Am J Public Health. 2010; 100:298–305. PMID:
20019314.

4. Park BH, Jung M, Lee TJ. Associations of income and wealth with health status in the Korean elderly. J Prev Med Public Health. 2009; 42:275–282. PMID:
19805999.

5. Ethisan P, Somrongthong R, Ahmed J, Kumar R, Chapman RS. Factors related to physical activity among the elderly population in rural Thailand. J Prim Care Community Health. 2017; 8:71–76. PMID:
27799413.

6. Amarantos E, Martinez A, Dwyer J. Nutrition and quality of life in older adults. J Gerontol A Biol Sci Med Sci. 2001; 56 Spec No 2:54–64. PMID:
11730238.

7. Seo S, Cho M, Kim Y, Ahn J. The relationships among satisfaction with food-related life, depression, isolation, social support, and overall satisfaction of life in elderly South Koreans. J Korean Diet Assoc. 2013; 19:159–172.

8. Patterson RE, Haines PS, Popkin BM. Diet quality index: capturing a multidimensional behavior. J Am Diet Assoc. 1994; 94:57–64. PMID:
8270756.

9. Haines PS, Siega-Riz AM, Popkin BM. The Diet Quality Index revised: a measurement instrument for populations. J Am Diet Assoc. 1999; 99:697–704. PMID:
10361532.
10. Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008; 108:1896–1901. PMID:
18954580.

11. Jankovic N, Geelen A, Streppel MT, de Groot LC, Orfanos P, van den Hooven EH, Pikhart H, Boffetta P, Trichopoulou A, Bobak M, Bueno-de-Mesquita HB, Kee F, Franco OH, Park Y, Hallmans G, Tjønneland A, May AM, Pajak A, Malyutina S, Kubinova R, Amiano P, Kampman E, Feskens EJ. Adherence to a healthy diet according to the World Health Organization guidelines and all-cause mortality in elderly adults from Europe and the United States. Am J Epidemiol. 2014; 180:978–988. PMID:
25318818.

12. Huijbregts P, Feskens E, Räsänen L, Fidanza F, Nissinen A, Menotti A, Kromhout D. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and The Netherlands: longitudinal cohort study. BMJ. 1997; 315:13–17. PMID:
9233319.

13. Lim J, Lee Y, Shin S, Lee HW, Kim CE, Lee JK, Lee SA, Kang D. An association between Diet Quality Index for Koreans (DQI-K) and total mortality in Health Examinees Gem (HEXA-G) study. Nutr Res Pract. 2018; 12:258–264. PMID:
29854332.

14. Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab. 2013; 20:1–10. PMID:
24524049.

15. Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006; 61:72–77. PMID:
16456196.

16. Yanai H. Nutrition for sarcopenia. J Clin Med Res. 2015; 7:926–931. PMID:
26566405.

17. Bloom I, Shand C, Cooper C, Robinson S, Baird J. Diet quality and sarcopenia in older adults: a systematic review. Nutrients. 2018; 10:308.

18. Chan R, Leung J, Woo J. A Prospective cohort study to examine the association between dietary patterns and sarcopenia in Chinese community-dwelling older people in Hong Kong. J Am Med Dir Assoc. 2016; 17:336–342. PMID:
26774365.

19. Brown JC, Harhay MO, Harhay MN. Physical activity, diet quality, and mortality among sarcopenic older adults. Aging Clin Exp Res. 2017; 29:257–263. PMID:
27020695.

20. Smee D, Pumpa K, Falchi M, Lithander FE. The Relationship between diet quality and falls risk, physical function and body composition in older adults. J Nutr Health Aging. 2015; 19:1037–1042. PMID:
26624217.

21. Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care. 2010; 33:1652–1654. PMID:
20460442.

22. Chin SO, Rhee SY, Chon S, Hwang YC, Jeong IK, Oh S, Ahn KJ, Chung HY, Woo JT, Kim SW, Kim JW, Kim YS, Ahn HY. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS One. 2013; 8:e60119. PMID:
23533671.

23. Kim KM, Lim S, Choi SH, Kim JH, Shin CS, Park KS, Jang HC. Cardiometabolic implication of sarcopenia: the Korea National Health and Nutrition Examination Study (KNHANES) 2008–2010. IJC Metab Endocr. 2014; 4:63–69.

24. Chung JY, Kang HT, Lee DC, Lee HR, Lee YJ. Body composition and its association with cardiometabolic risk factors in the elderly: a focus on sarcopenic obesity. Arch Gerontol Geriatr. 2013; 56:270–278. PMID:
23079031.

25. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Health ABC Study Investigators. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003; 51:1602–1609. PMID:
14687390.

26. Lee S, Lee JA, Kim JY, Zu Kim Y, Park HS. The risk factors of sarcopenia among Korean elderly men: based on 2009 Korean National Health and Nutrition Examination Survey Data. J Obes Metab Syndr. 2014; 23:23–31.
27. Kim SH, Kim TH, Hwang HJ. The relationship of physical activity (PA) and walking with sarcopenia in Korean males aged 60 years and older using the Fourth Korean National Health and Nutrition Examination Survey (KNHANES IV-2, 3), 2008–2009. Arch Gerontol Geriatr. 2013; 56:472–477. PMID:
23298535.

28. Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009; 12:86–90. PMID:
19057193.

29. Geirsdottir OG, Arnarson A, Ramel A, Jonsson PV, Thorsdottir I. Dietary protein intake is associated with lean body mass in community-dwelling older adults. Nutr Res. 2013; 33:608–612. PMID:
23890349.

30. Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, Doehner W, Fearon KC, Ferrucci L, Hellerstein MK, Kalantar-Zadeh K, Lochs H, MacDonald N, Mulligan K, Muscaritoli M, Ponikowski P, Posthauer ME, Rossi Fanelli F, Schambelan M, Schols AM, Schuster MW, Anker SD. Society for Sarcopenia, Cachexia, and Wasting Disease. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010; 11:391–396. PMID:
20627179.

31. Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznariç Z, Nair KS, Singer P, Teta D, Tipton K, Calder PC. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN expert group. Clin Nutr. 2014; 33:929–936. PMID:
24814383.

32. Zeng FF, Xue WQ, Cao WT, Wu BH, Xie HL, Fan F, Zhu HL, Chen YM. Diet-quality scores and risk of hip fractures in elderly urban Chinese in Guangdong, China: a case-control study. Osteoporos Int. 2014; 25:2131–2141. PMID:
24861906.

33. Chan R, Leung J, Woo J. Dietary patterns and risk of frailty in Chinese community-dwelling older people in Hong Kong: a prospective cohort study. Nutrients. 2015; 7:7070–7084. PMID:
26305253.
34. Jahangir A, Mirza M, Shahreyar M, Mengesha T, Shearer R, Sultan S, Jahangir A, Choudhuri I, Nangia V, Dhala A, Bhatia A, Niazi I, Sra J, Tajik AJ. Presence of obesity is associated with lower mortality in elderly patients with implantable cardioverter defibrillator. Int J Obes. 2018; 42:169–174.

35. Alkerwi A, Vernier C, Crichton GE, Sauvageot N, Shivappa N, Hébert JR. Cross-comparison of diet quality indices for predicting chronic disease risk: findings from the Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) study. Br J Nutr. 2015; 113:259–269. PMID:
25475010.

36. Ramírez-Vargas E, Arnaud-Viñas MR, Delisle H. Prevalence of the metabolic syndrome and associated lifestyles in adult males from Oaxaca, Mexico. Salud Publica Mex. 2007; 49:94–102. PMID:
17522735.

37. Milajerdi A, Namazi N, Larijani B, Azadbakht L. The Association of dietary quality indices and cancer mortality: a systematic review and meta-analysis of cohort studies. Nutr Cancer. 2018; 70:1091–1105. PMID:
30321058.

38. Zarrin R, Ibiebele TI, Marks GC. Development and validity assessment of a diet quality index for Australians. Asia Pac J Clin Nutr. 2013; 22:177–187. PMID:
23635360.
39. Chen LW, Fung SM, Fok D, Leong LP, Toh JY, Lim HX, Pang WW, Tan KH, Chong YS, Yap F, Godfrey KM, Lee YS, Chong MF. The development and evaluation of a diet quality index for Asian toddlers and its perinatal correlates: the GUSTO cohort study. Nutrients. 2019; 11:535.

40. Granic A, Mendonça N, Sayer AA, Hill TR, Davies K, Siervo M, Mathers JC, Jagger C. Effects of dietary patterns and low protein intake on sarcopenia risk in the very old: the Newcastle 85+ study. Clin Nutr. 2020; 39:166–173. PMID:
30709690.

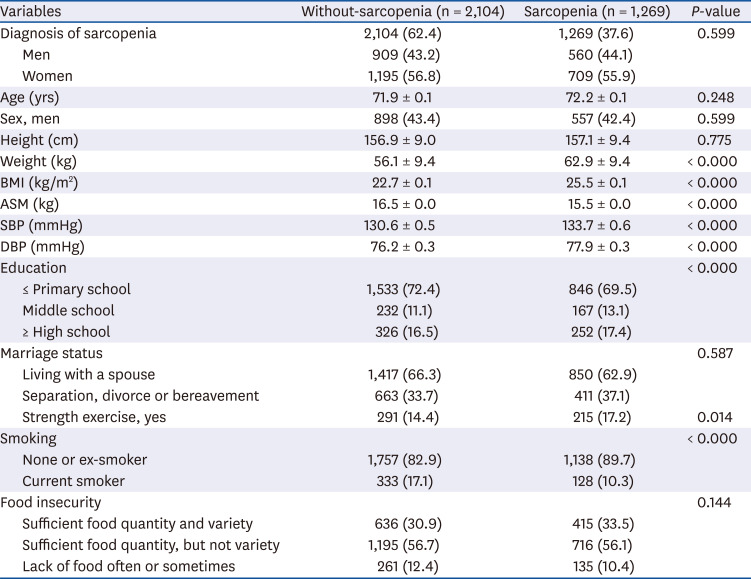
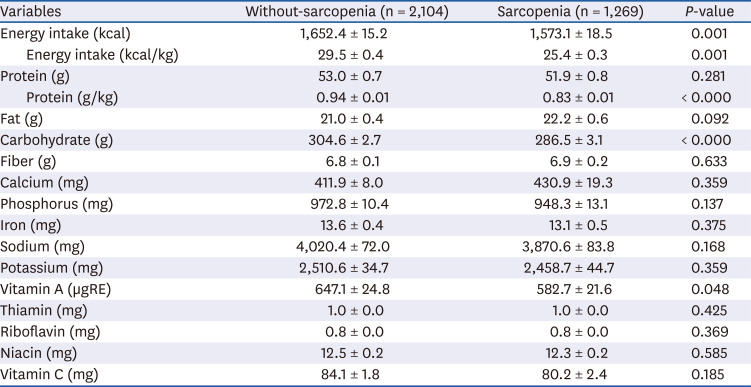
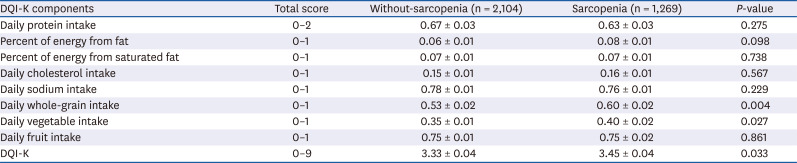
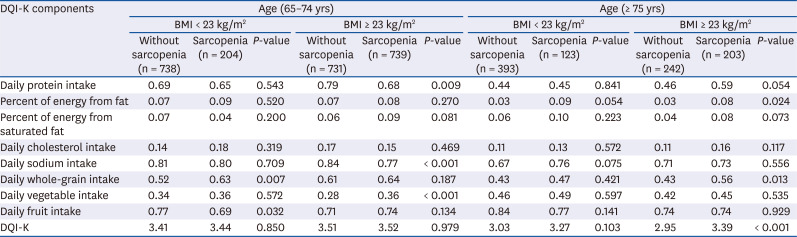




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download