This article has been
cited by other articles in ScienceCentral.
Abstract
Several lung transplantation (LTx) patients develop end-stage renal disease (ESRD) and often need a kidney transplant. Recently, the number of multiorgan transplantation cases has increased; however, no successfully combined redo lung-kidney transplantation has been reported in Korea. We present the first case of combined second redo lung-kidney transplantation in a patient with ESRD after LTx. In November 2018, a 40-year-old man with dyspnea was admitted to our hospital. Seventeen years ago, he underwent right pneumonectomy owing to refractory extensive drug-resistant tuberculosis. Four years ago, he underwent left single-LTx due to chronic respiratory failure. He was diagnosed with chronic lung allograft dysfunction and ESRD (glomerular filtration rate, <15). He underwent a second LTx that resulted in acute graft failure. Despite the empirical management, he was not responsive to treatment. He was required to use a home ventilator, but was able to maintain good muscle strength and to walk. However, regular dialysis was required. In January 2019, he underwent combined second redo lung-kidney transplantation and was discharged. At 1-year follow-up, his pulmonary and renal functions were stable without rejection. Combined lung-kidney transplantation could be an effective treatment for selective young patients with respiratory and renal failure who have undergone LTx.
Go to :

Keywords: Lung transplantation, Kidney transplantation, Multiorgan transplantation
|
HIGHLIGHTS |
Combined lung-kidney transplantation can offer the best possibility for cure in young patients with end-stage lung and kidney disease. Decisions must be made on a case-to-case basis considering patient- and donor-related factors.
|
Go to :

INTRODUCTION
Renal insufficiency is a common and significant lung transplantation (LTx) complication. Acute kidney injury (AKI) occurs in 52.5% of LTx, especially severe AKI requiring renal replacement therapy that occurs in 9.3% [
1]. Furthermore, stage IV chronic kidney disease (CKD) develops in approximately 8% of patients 1 year after transplantation, and the overall incidence of CKD after 5 years is reported to be 15.8%–68.6% [
2-
4]. Preoperative CKD is considered to be a risk factor for redo LTx [
5]. Combined lung-kidney transplantation can counteract these complications, but it should be performed only when kidney damage is irreversible.
Currently, although it is not frequently performed due to donor organ shortage, transplant centers worldwide are experiencing cases of simultaneously combined organ transplantations in adults (i.e., heart-lung, heart-kidney, and lung-kidney transplants). Conversely, the experience with combined lung-kidney transplantation has been limited. In the United States, only 65 cases were reported to the Organ Procurement and Transplantation Network from 1995 to 2019 [
6]. In Korea, only three lung-kidney transplantation cases were reported from 2000 to 2019 [
7]. Despite the few cases of lung-kidney transplantation, increasing demand for this procedure is expected as the number of lung transplantations increases. In addition, the number of redo transplant cases is also prevalent, mostly with younger recipients [
6,
7], thereby increasing the risk of renal insufficiency and the long-term use of calcineurin inhibitors, which can be an obstacle for redo lung transplantations. Therefore, lung-kidney transplantation is rare but necessary for select cases. To share this clinical experience, we report a rare case of combined second redo lung-kidney transplantation in a patient who developed ESRD after previous LTx.
The Institutional Review Board of Pusan National University Yangsan Hospital gave written approval for the study and waived the need for informed consent (05-2020-027).
Go to :

CASE REPORT
A 40-year-old man visited Pusan National University Yangsan Hospital complaining of dyspnea. Seventeen years ago, he underwent right pneumonectomy owing to refractory extensive drug-resistant tuberculosis. Four years prior, he underwent left single-LTx due to chronic respiratory failure. He developed AKI (creatinine, 1.36 mg/dL) 6 months after the first LTx and did not recover despite the reduced tacrolimus level from 12 to 10 ng/mL. The patient was evaluated for other possible causes of renal injury, including hemolytic uremia syndrome due to thrombotic microangioapathy secondary to calcineurin inhibitor use, other drug toxicities, contrast nephropathy, obstructive nephropathy secondary to nephrolithiasis, atheroembolism, and polyomavirus BK virus infection, but no abnormalities were noted. Despite stopping all possible nephrotoxic drugs, except for the immunosuppressants, and lowering the tacrolimus level from 10 to 8, he progressed to stage III CKD (creatinine, 1.54 mg/dL) at 1 year after first LTx. He could not use alternative immunosuppressants of mTOR inhibitors or interleukin-2 inhibitor to replace or minimize calcineurin inhibitors due to high cost; thus, he lowered the tacrolimus level from 8 to 6, and increased the doses of steroid and mycophenolate mofetil (MMF) to 30 mg and 1,500 mg, respectively. We aggressively treated the associated comorbidities that can worsen renal failure such as hypertension, diabetes, and hyperlipidemia. Despite all those efforts, he progressed to the stage IV CKD (creatinine, 2.67 mg/dL) at 32 months. At 39 months after first LTx, he had stopped taking his tacrolimus medication. He was diagnosed with chronic lung allograft dysfunction 11 months before his visitation to our hospital due to dyspnea, and was listed as a waiting recipient for a redo LTx. The patient was required home oxygen treatment (2 L/min). The initial blood test results at admission were as follows: hematocrit, 36.2%; creatinine, 4.56 mg/dL (glomerular filtration rate [GFR] <15); urea, 43.3 mg/dL; Na, 135 mEq/L; K, 4.8 mEq/L; and high-sensitivity C-reactive protein (blood), 9.38 mg/dL. The arterial blood gas results were as follows: pH, 7.39; pO
2, 56 mmHg; and pCO
2, 61 mmHg. The initial chest radiograph revealed left lower lung field consolidation (
Fig. 1A), although sputum culture was negative. Despite intravenous antibiotic treatment (piperacillin/tazobactam and levofloxacin) with mechanical ventilator support, carbon dioxide retention continued and his chest radiograph showed no improvement. On admission day 8, he underwent left single-LTx from a deceased donor. The lung transplant tissue pathology showed features of chronic lung allograft dysfunction (
Fig. 1B). He did not have any kind of donor-specific antibodies. He was administered with basiliximab and steroid as induction therapy in the same way as the first LTx, followed by steroids, MMF and tacrolimus as maintenance therapy.
 | Fig. 1(A) Chest radiograph showing right pneumonectomy and left lower lung field consolidation. (B) Pathology of the first lung transplantation tissue, showing pleuropulmonary fibrosis and intimal thickening of the large vessels, consistent with chronic lung allograft dysfunction. H&E, ×40. 
|
On postoperative day (POD) 7 of the redo LTx, multidrug-resistant
Acinetobacter baumannii and
Pseudomonas aeruginosa were detected in a bronchial wash specimen. Intravenous antibiotics (colistin and meropenem), prophylactic trimethoprim/sulfamethoxazole, and an inhaled antifungal agent (amphotericin B) were administered. However, acute lung rejection was suspected, which was subsequently treated with steroid pulse therapy (methylprednisolone 1,000 mg/day) for 3 days starting on POD 13. Due to recurrence of CO
2 retention, the patient could not be weaned from mechanical ventilation. On POD 19, continuous renal replacement therapy was initiated due to oliguria (urine output of <400 mL/day, GFR <15). On POD 21, a chest computed tomography scan was performed, which showed interval aggravation of acute lung rejection (
Fig. 2A), and a second round of steroid pulse therapy (methylprednisolone 1 g/day) was administered for 3 days. On POD 28, the patient developed fever with hypotension, and the blood cytomegalovirus polymerase chain reaction titer was elevated to 2,670 copies/mL; therefore, we added an antiviral agent (ganciclovir). Despite the empirical management of early graft dysfunction, he was not responsive to treatment and remained dependent on mechanical ventilation. He continued active rehabilitation at bedside, and aggressive nutritional therapy through a nasogastric tube (2,400 kcal/day). On POD 44 of the redo LTx, the continuous renal replacement therapy was converted to regular hemodialysis. He was required to maintain a home ventilator (pressure control mode, positive end-expiratory pressure of 4 cm H
2O, inspiratory positive airway pressure of 32 cm H
2O, respiratory rate of 22 breaths/min, and fraction of inspired oxygen of 35%), but was able to ambulate, thus maintained good muscle strength with a medical research council sum score of 57 points. We believed that he is in an irreversible state since he showed continuously deteriorating renal function since the first LTx and reached ESRD before the redo LTx. However, he was young with well-preserved muscle mass. We finally decided combined second redo LTx with simultaneous kidney transplantation. On POD 64 of the redo LTx, left single second redo LTx was performed followed by right kidney transplantation from a deceased donor. The dissection was performed without left lung ventilation under venovenous (VV) extracorporeal membrane oxygenation (ECMO) support because the patient was in the right pneumonectomy. The right femoral vein was used for venous drainage, and the right internal jugular vein for venous return. After the lung procurement has been confirmed to be safely completed from the donor procurement team, we switched the ECMO mode from VV to venoarterial for hemodynamic support. The femoral and internal jugular vein cannula was used as a drainage cannula, and a return cannula was inserted into the left femoral artery. In addition, a cannula was inserted to drain blood from the pulmonary artery. To reduce the ischemic time, lung extraction and bleeding control were completed before the donated lung arrived. The left lung transplant was performed immediately after the arrival of the lung, and then the kidney transplantation team performed the kidney transplantation. After the kidney transplantation, the chest and abdominal incision sites were closed and the operation was completed. For the postoperative lung care, ECMO was switched from VA to VV mode, and the patient was transferred to the intensive care unit. The total lung ischemic time was 162 minutes, and the kidney ischemic time was 250 minutes. After 12 hours, he was successfully weaned from VV ECMO. The pathology of the redo LTx tissue showed acute rejection, organizing pneumonia, and subpleural fibrosis (
Fig. 2B).
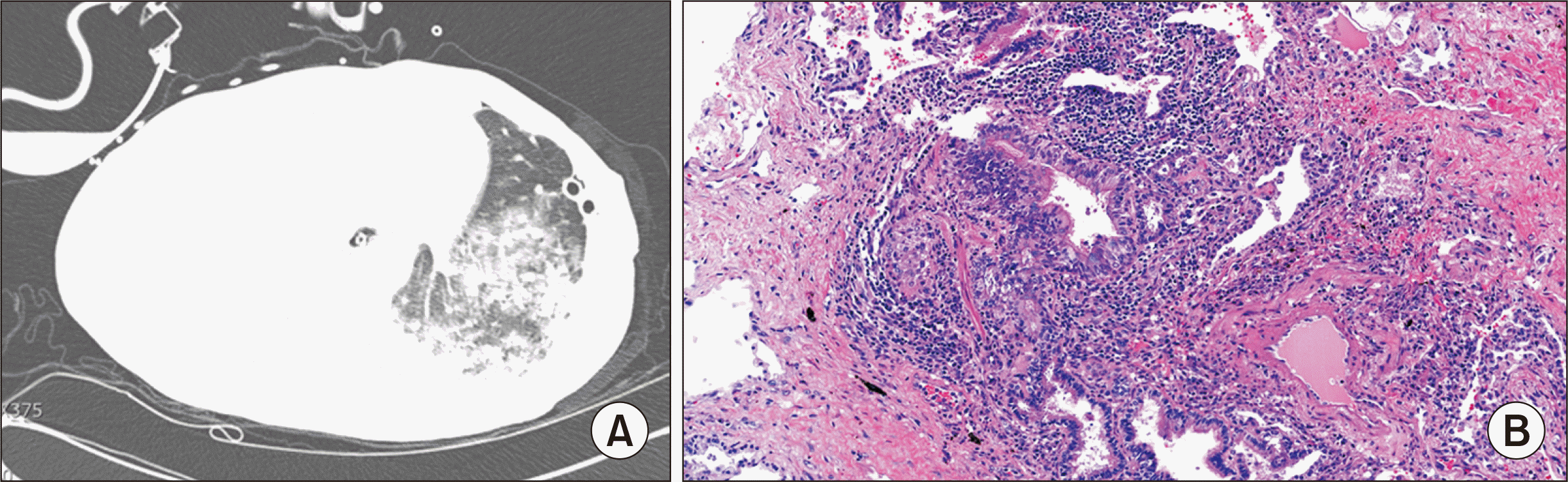 | Fig. 2(A) Chest computed tomography image obtained on postoperative day 21 after redo lung transplantation showing increased multiple patchy areas of consolidation and ground-glass opacity on the left lower lung basal segment with a small amount of left pleural effusion, which may indicate interval aggravation of acute rejection. (B) Pathology of re-lung transplantation tissue showing organizing pneumonia, acute cellular rejection, diffuse alveolar damage, and fibrosis. H&E, ×100. 
|
Since the combined second redo lung-kidney transplantation, bacterial pneumonia and acute pyelonephritis of the transplanted kidney occurred. The patient’s condition improved with the double-J stent insertion and antibiotic treatment. Fifty-five days after the combined lung-kidney transplantation, he was discharged from the hospital without ventilator support. At 1-year follow-up, he had stable pulmonary and renal functions without rejection.
Go to :

DISCUSSION
LTx remains the only therapeutic option for end-stage lung disease. However, despite 56 years of refinement of surgical techniques, donor and recipient management, immunosuppression protocols, and rejection surveillance, the conditional graft median survival is only 5.5–6 years [
8]. Chronic lung allograft dysfunction still remains the main cause of graft failure after LTx, and retransplantation is then required for additional life extension [
9-
11]. However, redo lung transplantations are reported less often than primary lung transplantations. To date, 1,452 redo LTx cases were reported in the United States from 1988 to 2019, and 14 cases were reported in Korea from 2000 to 2019 [
6,
7]. Cases of second redo lung transplantations have been rarely reported [
12,
13]; this is the first case that has been reported in Korea. Generally, redo lung transplantations are infrequent and controversial owing to technical challenges, ethical considerations, donor lung shortage, and their potentially inferior outcomes to primary lung transplantations [
10,
11]. However, the survival rates were improved in the most recent retransplant cohort, which was only slightly worse than those in primary transplantation cases [
14]. Recipients at the initial transplantation time, who are young like our patient, may eventually require retransplantation and even multiple transplantations. Therefore, this second redo LTx case may be controversial in terms of the appropriate donor lung allocation, which is in shortage, even with the desire to optimize outcomes for the individual patient. However, second redo LTx is currently the only feasible option to treat allograft failure [
12,
13].
Traditionally, LTx in patients with significant renal dysfunction is controversial. Allocating lungs to candidates with advanced renal disease is particularly risky given the known nephrotoxicity of the standard immunosuppressant regimen (calcineurin inhibitor) and the increased likelihood of accelerated renal deterioration after transplantation [
15-
17]. Combined lung-kidney or sequential kidney transplantation after LTx can be a solution [
18]. Otani et al. [
19] showed that combining kidney with lung transplantations significantly increased life expectancy, preserved lung function, and improved the quality of life. For lung-kidney transplantation, the waiting list mortality rate is high, and the posttransplant 5-year survival is similar to that of LTx alone [
20].
In the present case, although the patient had severe respiratory compromise and renal failure, he was young with well-preserved muscle mass. Except for ethical issues, our initial concern with second redo transplantation that might prove problematic in the reoperative setting was pleural adhesions, which often accompany massive intraoperative bleeding. An additional concern was the likelihood of acute rejection, given his shorter rejection-free survival period after redo LTx than that after his first transplantation. However, at 1-year follow-up, the patient showed no evidence of acute or chronic rejection. Although the patient showed good short-term outcomes, avoiding ethical criticisms in deciding retransplant to these patients is difficult.
In a previous report of a patient who underwent second redo LTx, a Maastricht category IV donor was used after cardiac death due to ethical concerns regarding appropriate organ allocation [
12]. The authors suggested that this ‘‘extended criteria’’ donor would not have otherwise been used. In this case, the donor allocated to the patient was belonging to standard criteria.
The current Korean Network for Organ Sharing allocation system does not preclude such redo LTx. In addition, the organ allocation controversy concerning retransplantation and combined multiorgan transplantation is still unresolved in Korea. Considering the organ shortage for end-stage organ diseases, efforts to reduce kidney injury after LTx seem to be more important and should be emphasized. However, in spite of all efforts, in young patients who have progressed to end-stage lung and kidney disease, combined lung-kidney transplantation can offer the best possibility for cure. Therefore, decisions must be made on a case-by-case basis with consideration of patient- and donor-related factors. Kidney transplantation should be considered when kidney dysfunction is irreversible.
To our knowledge, this is the first case report that describes a second redo LTx with a simultaneous kidney transplant in Korea. It demonstrates a feasible option with a good prognosis and no obvious early immune-mediated dysfunction. Although combined lung-kidney transplantation may be an acceptable therapeutic option for carefully selected patients with advanced, concomitant pulmonary and kidney disease, this case may be controversial owing to the fundamental ethical issue of donor shortage. Therefore, careful selection criteria should be discussed in depth within the transplant society regarding appropriate combined or redo transplant recipients.
Go to :

ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding/Support
This study was supported by research grant from the Ko­rean Society for Transplantation (2020-03-02001-007).
Author Contributions
Conceptualization: DK. Data curation: JN. Project administration: WHC. Visualization: JN. Writing–original draft: JN, WHC. Writing–review & editing: HJY.
Go to :

REFERENCES
1. Lertjitbanjong P, Thongprayoon C, Cheungpasitporn W, O'Corragain OA, Srivali N, Bathini T, et al. 2019; Acute kidney injury after lung transplantation: a systematic review and meta-analysis. J Clin Med. 8:1713. DOI:
10.3390/jcm8101713. PMID:
31627379. PMCID:
PMC6833042.

2. Monnier A, Krummel T, Collange O, Haffner G, Hirschi S, Dégot T, et al. 2015; Prevalence of acute and chronic renal failure after lung transplantation. J Heart Lung Transplant. 34 Suppl:S261. DOI:
10.1016/j.healun.2015.01.726. PMID:
32708858. PMCID:
PMC7404464.

3. Paradela de la Morena M, De La Torre Bravos M, Prado RF, Roel MD, Salcedo JA, Costa EF, et al. 2010; Chronic kidney disease after lung transplantation: incidence, risk factors, and treatment. Transplant Proc. 42:3217–9. DOI:
10.1016/j.transproceed.2010.05.064. PMID:
20970657.
5. Hook JL, Lederer DJ. 2012; Selecting lung transplant candidates: where do current guidelines fall short? Expert Rev Respir Med. 6:51–61. DOI:
10.1586/ers.11.83. PMID:
22283579. PMCID:
PMC3286653.

7. Korean Network for Organ Sharing (KONOS). 2016. 2018 Annual data report [Internet]. KONOS;Seoul: Available from:
http://konos.go.kr. cited 2019 Sep 20.
8. The International Society for Heart and Lung Transplantation (ISHLT). 2018. Overall Lung Transplantation Statistics [Internet]. ISHLT;Seoul: Available from:
https://ishlt.org. cited 2019 Sep 20.
10. Novick RJ, Stitt LW, Al-Kattan K, Klepetko W, Schäfers HJ, Duchatelle JP, et al. 1998; Pulmonary retransplantation: predictors of graft function and survival in 230 patients. Pulmonary Retransplant Registry. Ann Thorac Surg. 65:227–34. DOI:
10.1016/S0003-4975(97)01191-0. PMID:
9717912.
11. Brugière O, Thabut G, Castier Y, Mal H, Dauriat G, Marceau A, et al. 2003; Lung retransplantation for bronchiolitis obliterans syndrome: long-term follow-up in a series of 15 recipients. Chest. 123:1832–7. DOI:
10.1378/chest.123.6.1832. PMID:
12796157.
12. Oto T, Rowland M, Griffiths AP, Levvey BJ, Esmore DS, Williams TJ, et al. 2007; Third-time lung transplant using extended criteria lungs. Ann Thorac Surg. 84:642–4. DOI:
10.1016/j.athoracsur.2007.03.023. PMID:
17643651.

13. Vakil N, Mason DP, Yun JJ, Murthy SC, Budev MM, Pettersson GB. 2011; Third-time lung transplantation in a patient with cystic fibrosis. J Thorac Cardiovasc Surg. 141:e3–5. DOI:
10.1016/j.jtcvs.2010.09.028. PMID:
21092991.

14. Kawut SM, Lederer DJ, Keshavjee S, Wilt JS, Daly T, D'Ovidio F, et al. 2008; Outcomes after lung retransplantation in the modern era. Am J Respir Crit Care Med. 177:114–20. DOI:
10.1164/rccm.200707-1132OC. PMID:
17901410.

15. Peeters P, Van Laecke S, Vanholder R. 2007; Acute kidney injury in solid organ transplant recipients. Acta Clin Belg. 62 Suppl 2:389–92. DOI:
10.1179/acb.2007.087. PMID:
18284006.

16. Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, et al. 2013; The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report-2013; focus theme: age. J Heart Lung Transplant. 32:965–78. DOI:
10.1016/j.healun.2013.08.007. PMID:
24054805.

17. Weber NT, Bonani M, Benden C, Schleich A, Fehr T, Mueller TF, et al. 2018; Evolution of lung and kidney allograft function in patients receiving kidney after lung transplantation. Clin Transplant. 32:e13169. DOI:
10.1111/ctr.13169. PMID:
29194767.

18. Srinivas TR, Stephany BR, Budev M, Mason DP, Starling RC, Miller C, et al. 2010; An emerging population: kidney transplant candidates who are placed on the waiting list after liver, heart, and lung transplantation. Clin J Am Soc Nephrol. 5:1881–6. DOI:
10.2215/CJN.02950410. PMID:
20813856. PMCID:
PMC2974390.

19. Otani S, Levvey BJ, Westall GP, Paraskeva M, Whitford H, Williams T, et al. 2015; Long-term successful outcomes from kidney transplantation after lung and heart-lung transplantation. Ann Thorac Surg. 99:1032–8. DOI:
10.1016/j.athoracsur.2014.11.023. PMID:
25624053.

20. Cassuto JR, Reese PP, Sonnad S, Bloom RD, Levine MH, Olthoff KM, et al. 2010; Wait list death and survival benefit of kidney transplantation among nonrenal transplant recipients. Am J Transplant. 10:2502–11. DOI:
10.1111/j.1600-6143.2010.03292.x. PMID:
20977641. PMCID:
PMC2966021.

Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download