Abstract
Background
The present study intended to investigate the allocation status of pediatric deceased donor liver allografts. We analyzed the incidence of pediatric deceased donors in the Korean Network for Organ Sharing (KONOS) database and single-institutional status of liver transplantation (LT) using pediatric donors.
Methods
We assessed the nationwide incidence of pediatric donors aged ≤15 years and conducted single-center analysis of LT using pediatric donors.
Results
Between 2010 and 2019, pediatric donors aged ≤15 years accounted for 171 out of 4,395 donors (3.9%) in the KONOS database and 31 out of 640 liver donors (4.8%) in Asan Medical Center (AMC) database. In AMC, 11 (35.5%) and 20 (64.5%) grafts were allocated to pediatric recipients aged ≤15 years and adult recipients aged ≥19 years, respectively. All nine livers from donors aged ≤5 years were implanted in pediatric recipients aged ≤5 years. From 21 donors aged ≥9 years, 16 whole liver grafts and four split extended right liver grafts were implanted in 20 adult recipients and two split left lateral section grafts were implanted in two pediatric recipients. Four split liver grafts were implanted in other institutions. The overall patient survival rates at 1, 3, and 5 years were 90.9%, 80.8%, and 80.8%, respectively in pediatric-to-pediatric LT group and 69.6%, 58.4%, and 58.4%, respectively in pediatric-to-adult LT group (P=0.21).
Go to : 
Go to : 
Liver transplantation (LT) is an effective treatment for patients with end-stage liver disease due to its improved outcomes and broadening spectrum of indications. Shortage of organ donors and increased demand for LT have led to widening of concepts to increase the availability of liver grafts for LT. Even though old and marginal liver donors, living donors, and domino procedures have been accepted, a profound donor organ shortage is still encountered.
For pediatric patients, LT with size-matched whole liver allografts from pediatric donors would be ideal. However, a considerable proportion of pediatric donor liver allografts have been implanted in adult patients [1-3]. Moreover, the availability of detailed information regarding graft allocation is limited in Korea [4]. Thus, there is an essential need to analyze the allocation status of pediatric deceased donor liver allografts in Korea. We investigated the incidence of pediatric deceased donors in the Korean Network for Organ Sharing (KONOS) database and the status of LT using pediatric donors in a high-volume LT center.
Go to : 
The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2020-0857). The requirement for informed consent was waived by the Review Board due to the retrospective nature of this study. This study was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki 2013.
The purpose of this study was to investigate the allocation status of pediatric deceased donor liver allografts. This study comprised of two parts. The first part consisted of an assessment of the nationwide incidence of pediatric donors in Korea using the KONOS database. The second part consisted of a retrospective single-center analysis of LT using pediatric donors.
The study period was set between January 2010 and December 2019 for both parts of this study. We defined pediatric donors as donors aged ≤15 years. The recipients were divided into the pediatric-to-pediatric LT group (recipients aged ≤15 years) and the pediatric-to-adult LT group (recipients aged ≥19 years). Post-transplant outcomes of the two groups were compared. The recipients in this study were followed up until May 2020.
The numerical data were presented as mean±standard deviation. The continuous variables were compared using Student t-test. The incidence variables were compared using the chi-square test and the Fisher’s exact test. The survival rates were estimated using the Kaplan-Meier method and compared using a log-rank test. A P-value <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS ver. 22 (IBM Corp., Armonk, NY, USA).
Go to : 
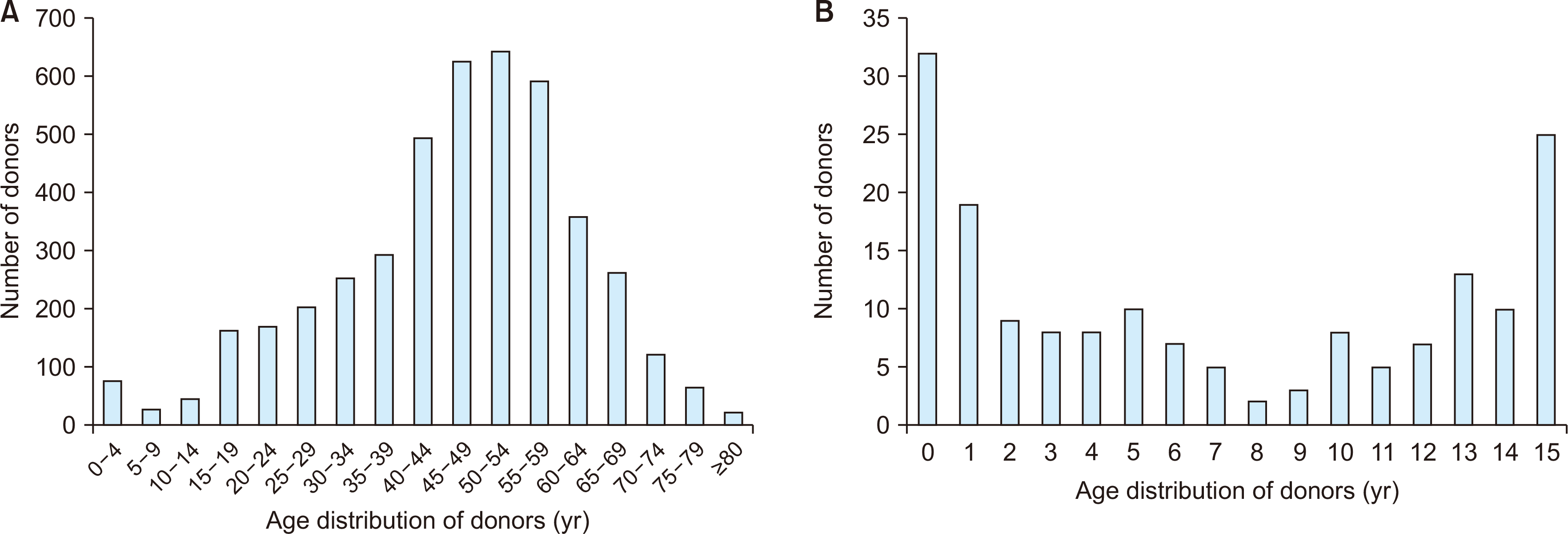
During the 10-year study period, the total number of all deceased organ donors was 4,395. Among these, 171 (3.9%) were pediatric donors aged ≤15 years. The distribution of donors according to age is depicted in Fig. 1.
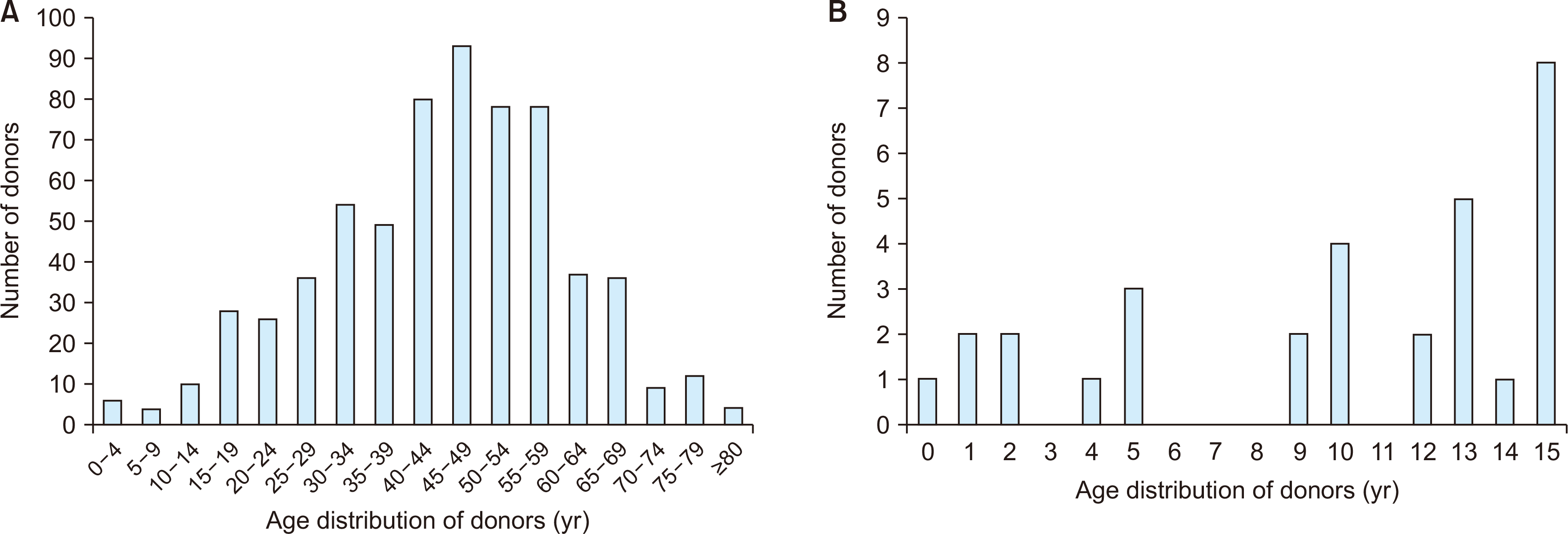
During the 10-year study period, 640 deceased donor liver transplantations (DDLTs) were performed at our institution and 31 (4.8%) DDLTs used pediatric donor liver allografts (Fig. 2). Among these 31 cases, there were 11 cases (35.5%) of pediatric-to-pediatric DDLT including one case of multivisceral transplantation and 20 cases (64.5%) of pediatric-to-adult DDLT including one case of liver-lung transplantation. All whole liver grafts from nine donors aged ≤5 years were implanted in pediatric recipients aged ≤5 years as whole liver grafts (n=8) and multivisceral transplantation (n=1). The mean weight of liver grafts was 451.3±111.4 g (range, 245–615 g) and the graft-recipient weight ratio (GRWR) was 3.83%±1.18% (range, 2.34– 5.75%).
From 21 donors aged ≥9 years, 16 whole liver grafts and four split extended right liver grafts were implanted in 20 adult recipients and two split left lateral section grafts were implanted in two pediatric recipients. One split extended right liver graft and three split left lateral section grafts were implanted in other institutions. The mean weight of liver grafts and the GRWR were 1,233.0±301.3 g (range, 596–1,620 g) and 2.08%±0.48% (range, 1.15%–2.84%), respectively in 16 adult whole liver recipients; 919.3±135.3 g (range, 800–1070 g) and 1.62%±0.29% (range, 1.23%–1.89%), respectively in four adult split liver recipients; and 361.5±68.6 g (range, 313–410 g) and 2.90%±0.85% (range, 2.30%–3.50%), respectively in two pediatric split liver recipients.
The donor and recipient characteristics of the two groups are presented in Table 1. DDLTs using pediatric liver grafts included primary DDLTs in 26 patients and retransplantations in five patients. In-hospital mortality occurred in one case (9.1%) of pediatric-to-pediatric DDLT and in four cases (20.0%) of pediatric-to-adult DDLT. Graft failure was the cause of in-hospital mortality in the case of pediatric-to-pediatric DDLT. In-hospital mortality following pediatric-to-adult DDLT was due to sepsis in two patients, intracranial hemorrhage in one patient, and pulmonary thromboembolism in one patient. Severe early graft dysfunction did not occur in these early mortality cases. The causes of late patient mortality were multi-organ failure in one case of pediatric-to-pediatric DDLT; and graft-versus-host disease in one case, pneumonia in one case, chronic rejection in one case, and recurrence of hepatocellular carcinoma in one case of pediatric-to-adult DDLT.
Comparison of patient profiles
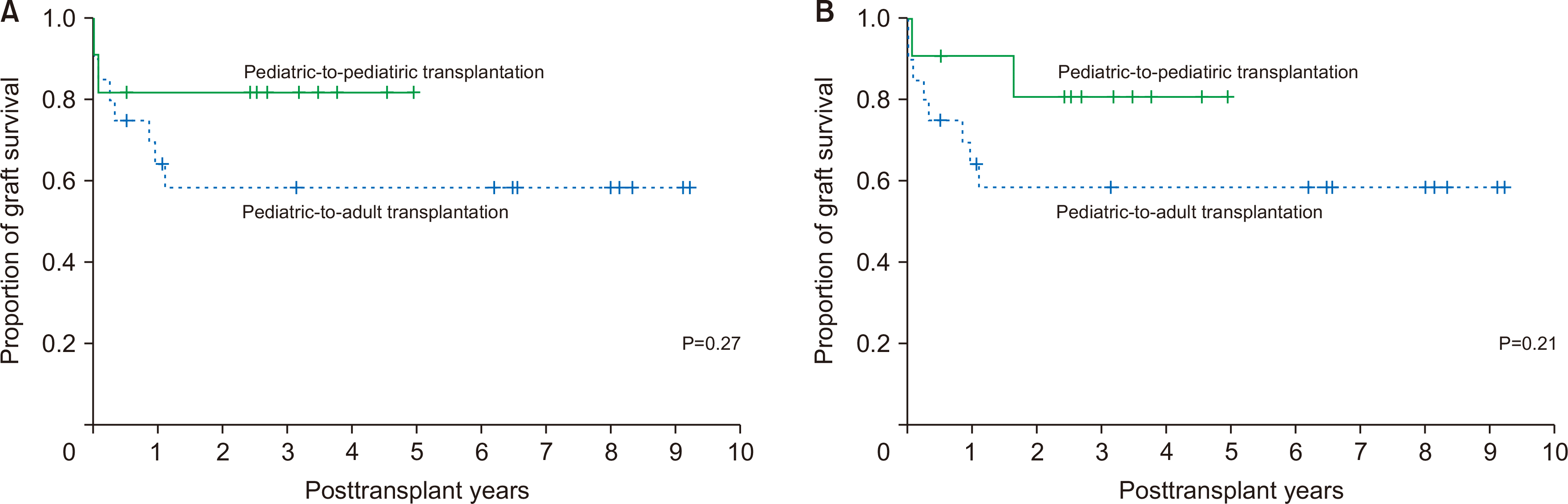
The graft survival rates at 1, 3, and 5 years were 81.8%, 81.8%, and 81.8%, respectively in the pediatric-to-pediatric DDLT group and 69.6%, 58.4%, and 58.4%, respectively in the pediatric-to-adult DDLT group (P=0.27) (Fig. 3A). There was only one case of pediatric liver retransplantation using a living donor liver graft following multivisceral transplantation at the third post-transplant day [5]. The overall patient survival rates at 1, 3, and 5 years were 90.9%, 80.8%, and 80.8%, respectively in the pediatric-to-pediatric DDLT group and 69.6%, 58.4%, and 58.4%, respectively in the pediatric-to-adult DDLT group (P=0.21) (Fig. 3B).
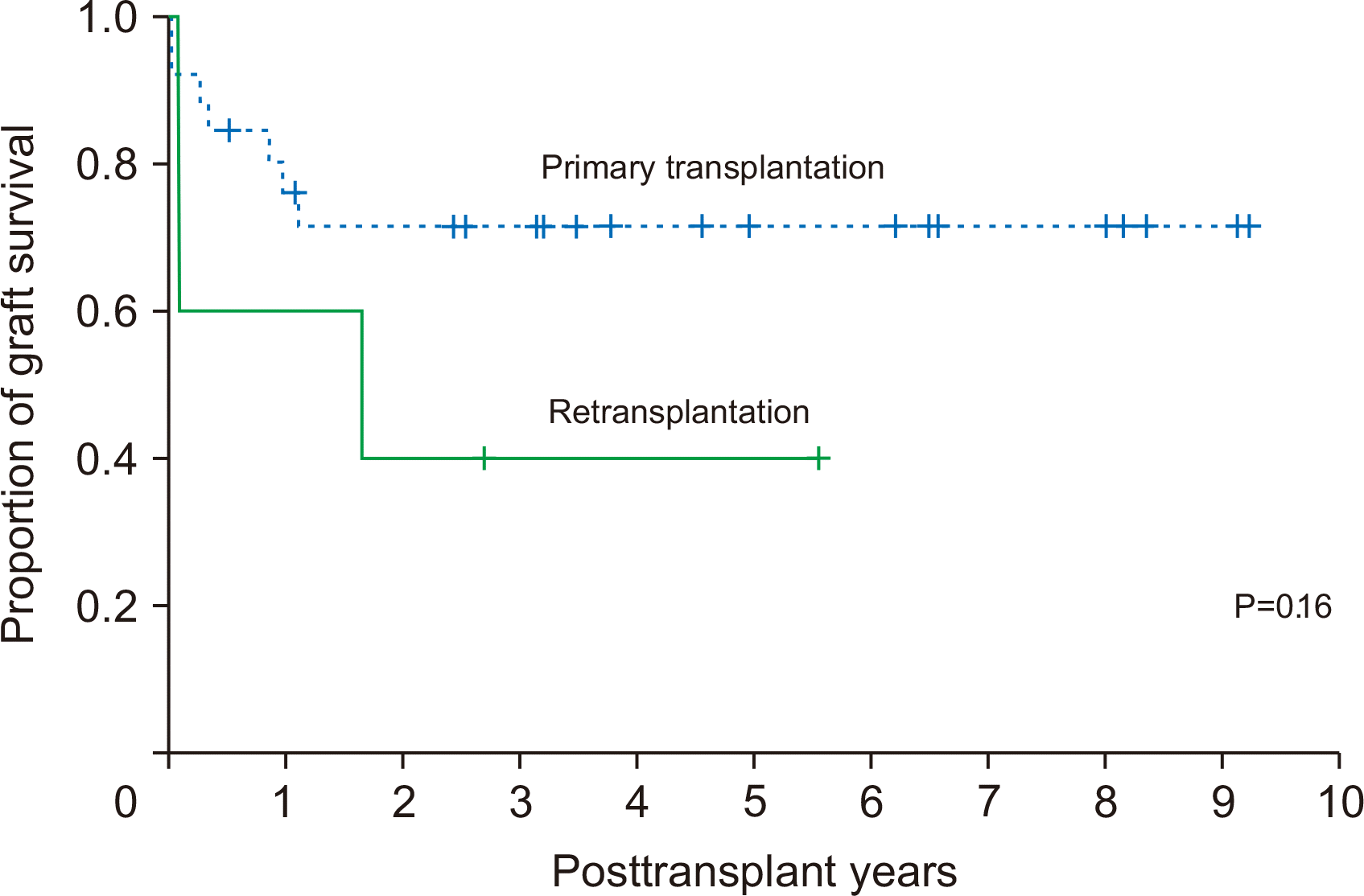
Retransplantation cases showed inferior survival outcomes when compared with primary DDLT with pediatric liver grafts (Fig. 4). However, the difference was not statistically significant (P=0.16), which may be attributed to the small sample size.
Go to : 
In Korea, 4,395 cases of deceased donors were reported during the 10-year period from 2010 to 2019. Among these, 171 (3.9%) were pediatric donors aged ≤15 years. In our institutional experience of DDLT during the same study period, there were 31 (4.8%) pediatric donors aged ≤15 years among 640 deceased donors used for DDLT. Among the 31 pediatric donor liver allografts, nine whole liver grafts were implanted in pediatric recipients, 16 whole liver grafts were implanted in adult recipients, and six split liver grafts were implanted in two pediatric patients and in four adult patients. Four split liver grafts were implanted in other institutions. Liver splitting was performed in five donors and these 10 split liver grafts were implanted in five pediatric patients and in five adult patients. Thus, livers from 31 pediatric donors were used for 14 pediatric patients and 21 adult patients. More than half of the pediatric donor liver grafts were allocated to adult patients probably due to donor-recipient body weight matching and lack of pediatric patients with high priority on the pediatric waiting list.
Pediatric donors aged up to 5 years usually have body weights below 20 kg. Liver allografts from these donors cannot be used for adult patients. Thus, all allografts from such donors were allocated to body weight-matched pediatric patients. According to the Korean standardized growth patterns of children, the 50th percentiles of body weight at 60 months, 120 months, and 180 months are 19.0 kg, 35.5 kg, and 60.1 kg, respectively. Thus, pediatric donors aged over 10 years have body weights comparable to adult patients. According to the KONOS regulations of donor-recipient body weight match ratio of 1:2, livers from these pediatric donors can be allocated to adult recipients as whole or split liver grafts. To the best of our knowledge, there is no KONOS regulation for the allocation of pediatric liver allografts to pediatric recipients on priority.
In pediatric patients aged below 5 years, graft-recipient size matching to avoid large-for-size graft implantation is one of the major concerns. The main problems of large-for-size liver grafts include the risk of abdominal compartment syndrome due to the small abdominal cavity of the recipient, discrepancies in vessel size, and insufficient portal circulation and tissue oxygenation [6,7]. A 25-month-old pediatric recipient in the present study underwent graft size reduction through in situ resection of the left lateral section, reducing the GRWR from 7.2% to 6.2%.
On the other hand, for adult and adolescent recipients, implantation of a small-for-size graft (e.g., living donor LT) can also be a matter of concern. In Taiwan, a 60-year-old woman with body weight of 56 kg received a whole liver graft with a GRWR of 0.46% from a 10-year-old donor weighing 12.8 kg [2]. Such an extreme mismatch of graft and body weight is not permitted in Korea according to the KONOS regulations. In the present study, the mean GRWR was 2.08%±0.48% in 16 pediatric-to-adult whole liver recipients and 1.62%±0.29% in four pediatric-to-adult split liver recipients.
The incidence of vascular complications reported in the pediatric LT literature is variable and can be up to 25%–33% [8-10]. Hepatic artery thrombosis is the most serious complication after LT and early hepatic artery thrombosis is the main cause of graft loss in pediatric LT. A similar incidence of early vascular complications was reported in the pediatric-to-pediatric LT group and in the pediatric-to-adult LT group [3]. Moreover, low body weight of the recipient was an independent risk factor for vascular complications in pediatric LT [3]. Vascular complications frequently occur following implantation of a whole liver graft in an infant recipient due to the small vessel size, even when the graft size is well matched with the recipient body size. We previously reported that portal vein complications occurred in four out of seven cases of infant-to-infant whole LT and these complications could be successfully prevented through customized surgical techniques with side-to-side unification venoplasty [11].
In the present study, the pediatric end-stage liver disease (PELD) scores in pediatric patients were much lower than the model for end-stage liver disease (MELD) scores in adult patients. Considering the characteristics of liver diseases in childhood, PELD scores in pediatric patients cannot be compared directly with MELD scores in adult patients in the current Korean setting. Severely abnormal liver function is not observed in many grave conditions that require LT in children. Inborn errors of metabolism such as urea cycle disorders or organic acidemia and hepatoblastoma are examples of such conditions [4]. Therefore, we suggest that liver allografts from pediatric donors aged ≤12 years (age limit of PELD score) should be allocated to pediatric recipients on priority.
The Organ Procurement and Transplantation Network (OPTN) of North America clearly prioritizes potential pediatric transplant recipients while allocating livers from pediatric deceased donors [12]. The ethical principles behind their pediatric organ allocation policy are elucidated by the Pediatric Transplantation Committee and the Ethics Committee of the OPTN/United Network for Organ Sharing [13]. The National Organ Transplant Act charges the OPTN to recognize the differences in health and organ transplantation issues between children and adults throughout the system and to adopt criteria, policies, and procedures that address the unique health care needs of children.
The present study has several notable limitations. It was a retrospective, single-center study with a relatively small number of patients. The detailed allocation status of pediatric donors recorded in the KONOS database was not available. Further high-volume multicenter studies are necessary to validate the results of the present study.
In conclusion, the results of the present study suggested that more than half of the pediatric donor liver allografts were allocated to adult patients. It is necessary to revise the recipient criteria for the allocation of livers from deceased donors, especially those from pediatric donors, to address the special needs of children on the pediatric LT waiting list.
Go to : 
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding/Support
This study was supported by research grant from the Ko­rean Society for Transplantation (2020-03-01003-005).
Author Contributions
Conceptualization: SH. Data curation: DYK, KMK, SHO, SGL. Methodology: CSA, KHK, DBM, TYH, GWS, DHJ, GCP. Visualization: SH. Writing–original draft: SH, JMN. Writing–review & editing: SH.
Go to : 
REFERENCES
1. Emre S, Soejima Y, Altaca G, Facciuto M, Fishbein TM, Sheiner PA, et al. 2001; Safety and risk of using pediatric donor livers in adult liver transplantation. Liver Transpl. 7:41–7. DOI: 10.1053/jlts.2001.20940. PMID: 11150421.

2. Feng AC, Liao CY, Fan HL, Chen TW, Hsieh CB. 2015; A successful child-to-adult deceased donor liver transplantation: a case report and literature review. Ann Transplant. 20:21–4. DOI: 10.12659/AOT.893101. PMID: 25582243. PMCID: PMC4998643.

3. Zhang R, Zhu ZJ, Sun LY, Wei L, Qu W. 2018; Outcomes of liver transplantation using pediatric deceased donor livers: a single-center analysis of 102 donors. Chin Med J (Engl). 131:677–83. DOI: 10.4103/0366-6999.226901. PMID: 29521290. PMCID: PMC5865313.
4. Lee S, Lee SK. 2019; Pediatric liver transplantation in Korea: long-term outcomes and allocations. J Korean Soc Transplant. 33:1–5. DOI: 10.4285/jkstn.2019.33.1.1.

5. Hwang S, Kim DY, Namgoong JM, Kim KM, Oh SH, Kim KH, et al. 2020; Living donor liver retransplantation for primary non-function of liver graft following multivisceral transplantation in a pediatric patient. Ann Hepatobiliary Pancreat Surg. 24:198–202. DOI: 10.14701/ahbps.2020.24.2.198. PMID: 32457267. PMCID: PMC7271102.

6. Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, et al. 1999; Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 67:321–7. DOI: 10.1097/00007890-199901270-00024. PMID: 10075602.
7. Vanatta JM, Esquivel CO. 2007; Status of liver transplantation in infants < 5 kg. Pediatr Transplant. 11:5–9. DOI: 10.1111/j.1399-3046.2006.00627.x. PMID: 17328158.
8. Heffron TG, Welch D, Pillen T, Fasola C, Redd D, Smallwood GA, et al. 2005; Low incidence of hepatic artery thrombosis after pediatric liver transplantation without the use of intraoperative microscope or parenteral anticoagulation. Pediatr Transplant. 9:486–90. DOI: 10.1111/j.1399-3046.2005.00327.x. PMID: 16048601.

9. Shackleton CR, Goss JA, Swenson K, Colquhoun SD, Seu P, Kinkhabwala MM, et al. 1997; The impact of microsurgical hepatic arterial reconstruction on the outcome of liver transplantation for congenital biliary atresia. Am J Surg. 173:431–5. DOI: 10.1016/S0002-9610(97)00066-4. PMID: 9168083.

10. Ooi CY, Brandão LR, Zolpys L, de Angelis M, Drew W, Jones N, et al. 2010; Thrombotic events after pediatric liver transplantation. Pediatr Transplant. 14:476–82. DOI: 10.1111/j.1399-3046.2009.01252.x. PMID: 19849808. PMCID: PMC7520663.

11. Namgoong JM, Hwang S, Ahn CS, Jung DH, Park GC. 2020; Side-to-side portal vein reconstruction for infant-to-infant deceased donor whole liver transplantation: report of 2 cases with video. Ann Hepatobiliary Pancreat Surg. 24:301–4. DOI: 10.14701/ahbps.2020.24.3.301. PMID: 32843595. PMCID: PMC7452795.

12. United Network for Organ Sharing (UNOS). 2011. Organ distribution: allocation of livers [Internet]. UNOS;Richmond, VA: Available from: https://optn.transplant.hrsa.gov. cited 2020 Jun 30.
13. United Network for Organ Sharing (UNOS). 2011. Ethical principles of pediatric organ allocation [Internet]. UNOS;Richmond, VA: Available from: https://optn.transplant.hrsa.gov. cited 2020 Jun 30.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download