CASE REPORT
A 19-year-old female student, with underlying right renal dysplasia, was diagnosed with end-stage renal disease and started on continuation ambulatory peritoneal dialysis for 10 years until she received a kidney, donated by her mother on January 15, 2019. The patient had a moderate CMV risk, given both she and her mother showed positive CMV immunoglobulin G serology. She had normal immunological risk hence she was given 20 mg of basiliximab as induction agent for her renal transplantation. Unfortunately, her transplant graft was complicated by arterial thrombosis and required re-exploration and re-anastomosis. A double J-stent was inserted intraoperatively using a retrograde approach. Postoperatively, the patient recovered graft function immediately, but acquired an extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) driven septicemia, which required treatment with intravenous (IV) antibiotic carbapenem for 2 weeks. On January 31, 2019, the patient was discharged while on a triple immunosuppression (IS) regimen involving: 30 mg corticosteroid once per day, 6.5 mg tacrolimus twice per day, and 720 mg mycophenolate sodium (MPS) twice per day. At the time of discharge, the patient’s creatinine level was 77 µmol/L, with an average tacrolimus level of 10 ng/mL. Her double J-stent was removed on postoperative day 14 using a flexible cystoscope.
After 1 month, the patient was readmitted due to an increased creatinine level (129 µmol/L). The ultrasound of her graft kidney revealed moderate hydronephrosis although the renal mercaptoacetyltriglycine-3 scan showed no obstruction. A renal allograft biopsy was performed, showing mild to moderate tubulitis (t1–t2) with i1 changes. Her donor-specific antibody (DSA) was negative. The patient was treated as a borderline kidney rejection case and given pulse corticosteroids in the form of IV methylprednisolone, 1 g once per day, administered over 3 days. In the month that followed, the patient developed a urinary tract infection, which was resolved with an IV antibiotic. However, her kidney function continued to deteriorate (creatinine level, 186 µmol/L). A cystourethrogram was performed, which showed no reflux nephropathy changes.
Next, a renal graft biopsy was attempted but failed due to gross hydronephrosis. The patient underwent antegrade pyelogram and nephrostomy, which improved her kidney function (serum creatinine level decreased from 319 to 156 µmol/L). Given the unexplained hydronephrosis, further exploration was planned and the patient consequently underwent rigid cystoscopy, open exploration, ureteric excision, and ureteroneocystostomy in the 4th month after transplant. Intraoperatively, an extraluminal distal ureteric stricture was revealed, and the tissue biopsy findings of the ureter indicated a suture granuloma that stained negative for simian virus 40 (SV40).
At the same time, elevated BKV-nucleic acid titer (BKV-NAT) and CMV-NAT were detected (391 and 241 IU/mL, respectively) during the regular 2-weekly CMV titer and 4-weekly BKV titer monitoring, performed as a pre-emptive therapy approach. The patient was not on antiviral drugs as her CMV risk was being managed using a pre-emptive approach. Her MPS dosage was consequently reduced to 360 mg twice per day from 540 mg twice per day, after which her CMV-NAT became undetectable. Given the patient’s BKV-NAT was persistently elevated (14,743 to 22,088 IU/mL), the MPS dose was further reduced to 180 mg twice per day and, ultimately, withheld. After 1 week, the patient’s creatinine level increased to 172 µmol/L.
After conducting a renal allograft ultrasound, it was found that the previous hydronephrosis had resolved and the patient’s DSA was negative. A renal allograft biopsy, which included a total of 13 glomeruli and five interlobular arteries, was repeated. The histopathological examination (HPE) revealed severe tubulitis (t3) and severe interstitial lymphocytic infiltrates mixed with some plasma cells (20%; i3) (
Fig. 1). The background showed severe interstitial fibrosis and tubular atrophy (ct3, ci3) of 70%–80%. Moderate peritubular capillaritis (PTC2) was seen in combination with mild glomerulitis, but without active glomerular lesions or intimal vasculitis. In addition to observing scattered peritubular neutrophils, a few tubules containing granular casts with neutrophil exudates were also seen.
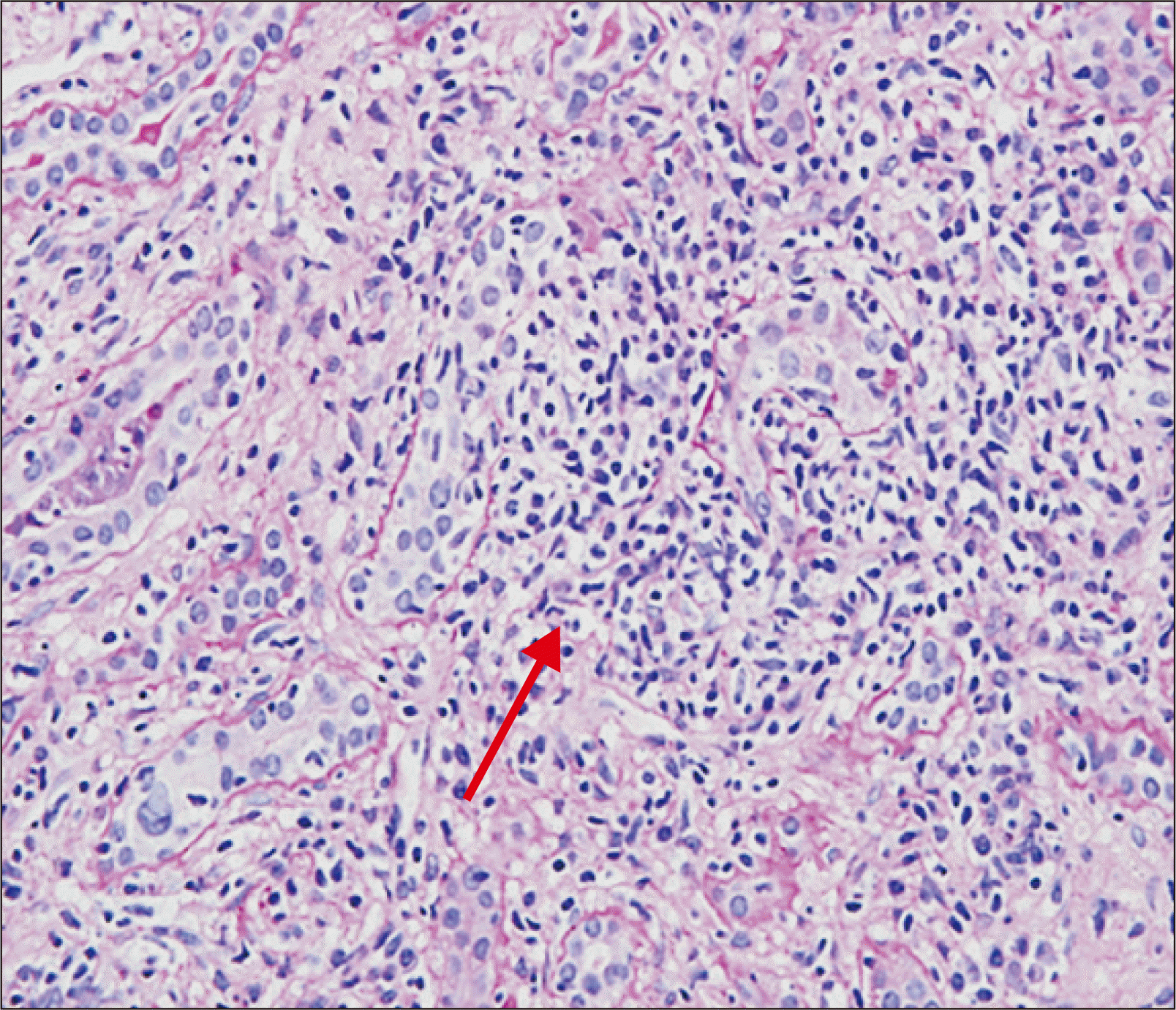 | Fig. 1H&E staining image at 4 months after transplant: severe tubulitis (T3) and interstitial lymphocytic infiltration in more than 50% of unscarred cortical parenchyma (i3; red arrow; magnification, ×40). 
|
We noticed observed typical CMV inclusion bodies that exhibited macronuclei and cytoplasmic eosinophilic inclusions on hematoxylin and eosin staining. Immunohistochemistry for CMV was also performed and confirmed CMV nephritis (
Fig. 2). Other signs of CMV driven extrarenal tissue invasion, including CMV retinitis, were not detected. Furthermore, enlarged tubular nuclei were observed, which indicated SV40 positivity, as confirmation of BKV nephritis. The BKV inclusions were observed far apart from the CMV inclusions in the HPE (
Fig. 3).
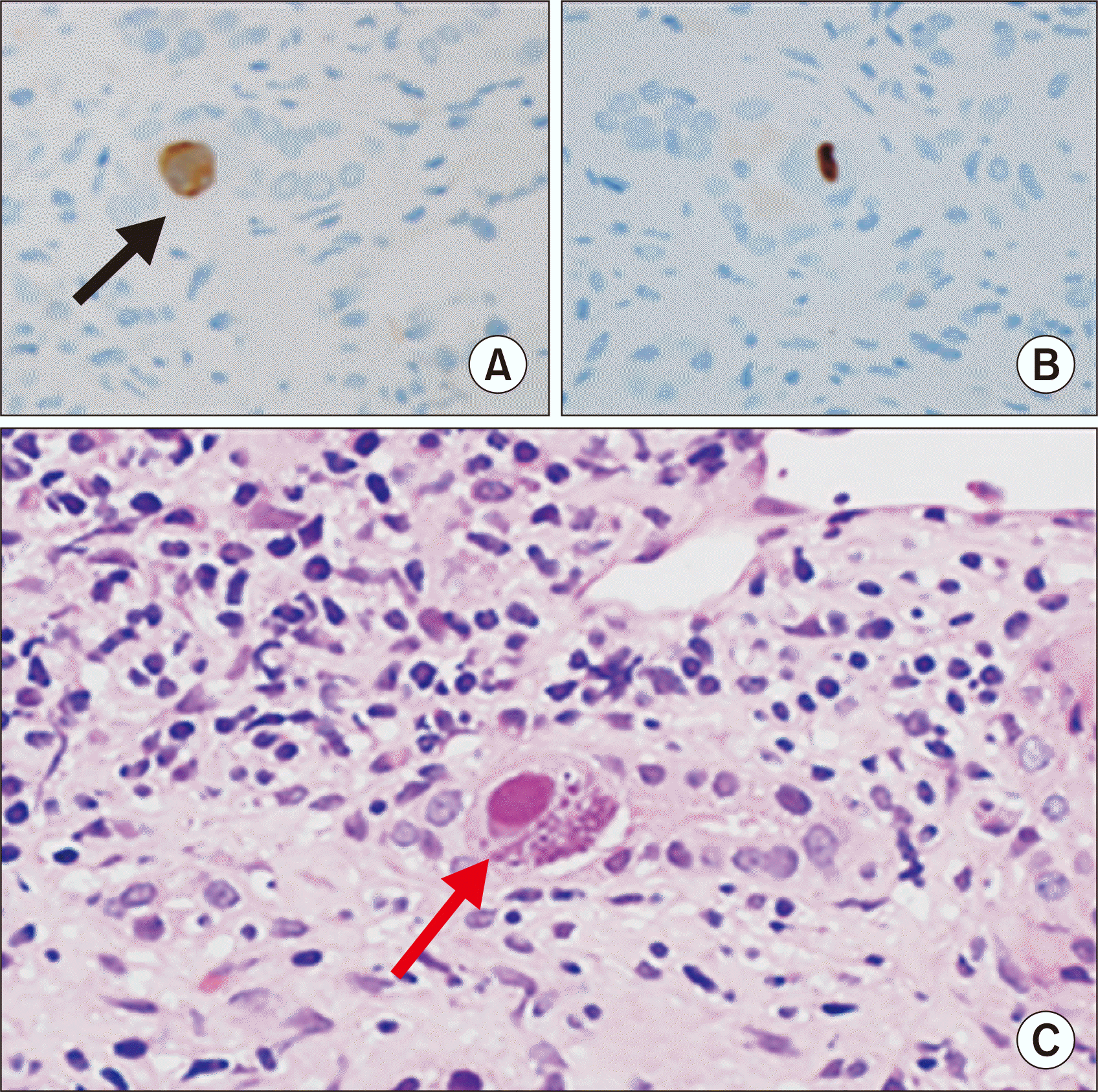 | Fig. 2Cytomegalovirus (CMV) inclusion body noted on immunohistochemical analysis (black arrow) at 4 months after transplantation. A typical CMV inclusion bodies (red arrow) with H&E staining. (A, B) CMV inclusion body with immunohistochemical staining. (C) CMV inclusion body with H&E staining (magnification, ×400). 
|
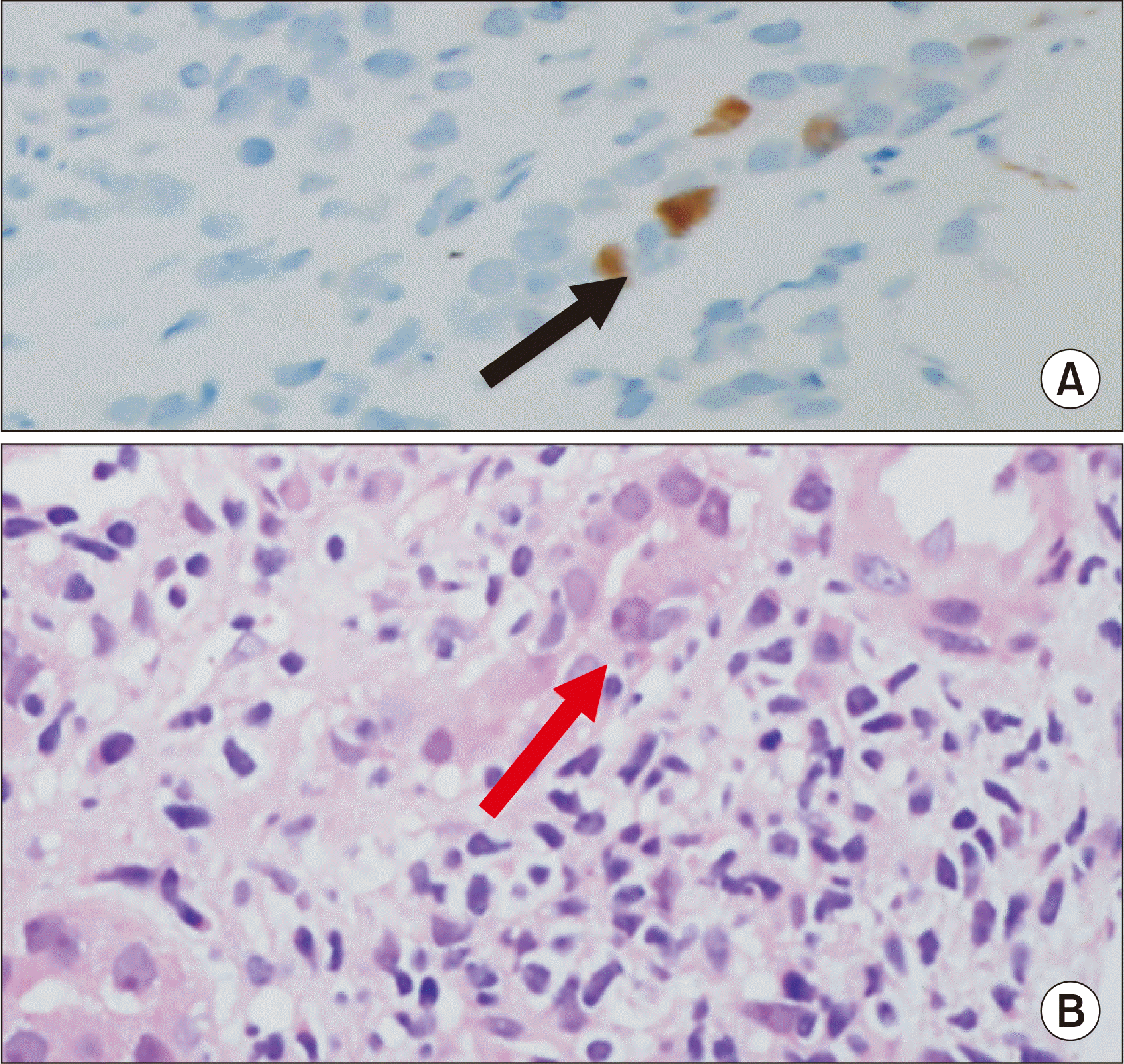 | Fig. 3Enlargement of the tubular nuclei (red arrow) with severe tubulointerstitial inflammation, without typical BK polyomavirus inclusion bodies noted at 4 months after transplantation. However, SV-40 positivity is observed (black arrow). (A) Black arrow shows SV-40 stain positive. (B) Red arrow shows enlargement of the tubular nuclei with background of severe tubulointerstitial inflammation. Magnification, ×400. 
|
Since the allograft renal biopsy showed severe interstitial fibrosis and tubular atrophy, oral everolimus, at 1.5 mg twice per day, was added to minimize tacrolimus exposure. Both tacrolimus and everolimus levels were at the targeted blood levels of 5 ng/mL. Subsequently, the patient’s BKV-NAT reduced to 2,575 IU/mL while her CMV-NAT remained undetectable (creatinine level, 137 µmol/L).
Additionally, the patient was treated with IV Ertapenem for acute pyelonephritis caused by ESBL-EC. Oral valganciclovir was administered over 3 weeks for CMV nephritis and IV immunoglobulin (IVIg), at 1 g/kg, was started and administered over 3 days for BKV nephritis. The patient’s kidney function partially improved (creatinine level, 166 µmol/L) and she was subsequently discharged, without any clinical evidence of rejection, with a follow-up appointment for the second course of IVIg after 1 month.
Go to :

DISCUSSION
Concurrent CMV and BKV infections in patient who underwent allograft kidney transplantation are extremely rarely encountered [
2,
3]. In this case, CMV infection was present as a localized disease and its existence may have accelerated the process of reactivation of BKV. This explains the BKV titer being persistently high, despite timely tapering off of the IS in this patient. The renal histopathological changes in CMV and BKV nephropathy may mimic cellular rejection, thus, posing both diagnostic and management challenges. The definitive criteria for distinguishing interstitial nephritis due to CMV and BKV from acute cellular rejection, however, has not been established [
3]. The presence of CMV associated inclusion bodies in the renal biopsy samples is unquestionably direct evidence of tissue invasion or CMV nephropathy. However, these inclusions are only detected in 1% of the transplant biopsy samples in patients with CMV disease [
1].
Conversely, BKV is the most common viral pathogen found in renal allografts [
4]. The most significant clinical sign of a BKV infection is graft dysfunction, in addition to other less common presentations, including ureteric strictures and hemorrhagic cystitis [
5]. A recent study also described an association between BKV nephritis and increased incidence of malignancy in patients with renal allografts [
6]. In this case, given the high index of suspicion, a thorough examination of histopathological specimens was required to establish a precise diagnosis. The concurrent infections should be aggressively managed since the infection are associated with poor allograft outcome [
7,
8].
Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download