1. Iansante V, Mitry RR, Filippi C, Fitzpatrick E, Dhawan A. 2018; Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr Res. 83:232–40. DOI:
10.1038/pr.2017.284. PMID:
29149103.

2. Soltys KA, Soto-Gutiérrez A, Nagaya M, Baskin KM, Deutsch M, Ito R, et al. 2010; Barriers to the successful treatment of liver disease by hepatocyte transplantation. J Hepatol. 53:769–74. DOI:
10.1016/j.jhep.2010.05.010. PMID:
20667616. PMCID:
PMC2930077.

3. Donato MT, Lahoz A, Montero S, Bonora A, Pareja E, Mir J, et al. 2008; Functional assessment of the quality of human hepatocyte preparations for cell transplantation. Cell Transplant. 17:1211–9. DOI:
10.3727/096368908787236620. PMID:
19181215.

4. Strom SC, Chowdhury JR, Fox IJ. 1999; Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis. 19:39–48. DOI:
10.1055/s-2007-1007096. PMID:
10349682.

5. Ho CM, Chen YH, Chien CS, Ho YT, Ho SL, Hu RH, et al. 2015; Transplantation speed offers early hepatocyte engraftment in acute liver injured rats: a translational study with clinical implications. Liver Transpl. 21:652–61. DOI:
10.1002/lt.24106. PMID:
25821041.

6. Riordan SM, Williams R. 2000; Acute liver failure: targeted artificial and hepatocyte-based support of liver regeneration and reversal of multiorgan failure. J Hepatol. 32(1 Suppl):63–76. DOI:
10.1016/S0168-8278(00)80416-X. PMID:
10728795.

7. Zhang B, Inagaki M, Jiang B, Miyakoshi M, Arikura J, Ogawa K, et al. 2009; Effects of bone marrow and hepatocyte transplantation on liver injury. J Surg Res. 157:71–80. DOI:
10.1016/j.jss.2008.12.013. PMID:
19345373.

8. Gómez-Aristizábal A, Ng C, Ng J, Davies JE. 2012; Effects of two mesenchymal cell populations on hepatocytes and lymphocytes. Liver Transpl. 18:1384–94. DOI:
10.1002/lt.23500. PMID:
22753359.

9. van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, et al. 2008; Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 47:1634–43. DOI:
10.1002/hep.22236. PMID:
18395843.
10. Balber AE. 2011; Concise review: aldehyde dehydrogenase bright stem and progenitor cell populations from normal tissues: characteristics, activities, and emerging uses in regenerative medicine. Stem Cells. 29:570–5. DOI:
10.1002/stem.613. PMID:
21308868.

11. Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. 2007; Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 104:11002–7. DOI:
10.1073/pnas.0704421104. PMID:
17569781. PMCID:
PMC1891813.

13. Gómez-Aristizábal A, Keating A, Davies JE. 2009; Mesenchymal stromal cells as supportive cells for hepatocytes. Mol Ther. 17:1504–8. DOI:
10.1038/mt.2009.158. PMID:
19584815. PMCID:
PMC2835270.

14. Yagi H, Parekkadan B, Suganuma K, Soto-Gutierrez A, Tompkins RG, Tilles AW, et al. 2009; Long-term superior performance of a stem cell/hepatocyte device for the treatment of acute liver failure. Tissue Eng Part A. 15:3377–88. DOI:
10.1089/ten.tea.2008.0681. PMID:
19397469. PMCID:
PMC2792056.

15. Meier RP, Müller YD, Morel P, Gonelle-Gispert C, Bühler LH. 2013; Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 11:1348–64. DOI:
10.1016/j.scr.2013.08.011. PMID:
24090934.

16. Liu WH, Song FQ, Ren LN, Guo WQ, Wang T, Feng YX, et al. 2015; The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J Cell Mol Med. 19:511–20. DOI:
10.1111/jcmm.12482. PMID:
25534251. PMCID:
PMC4369809.

17. Kanazawa H, Fujimoto Y, Teratani T, Iwasaki J, Kasahara N, Negishi K, et al. 2011; Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One. 6:e19195. DOI:
10.1371/journal.pone.0019195. PMID:
21559442. PMCID:
PMC3084802.

18. Li J, Zhang L, Xin J, Jiang L, Li J, Zhang T, et al. 2012; Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatology. 56:1044–52. DOI:
10.1002/hep.25722. PMID:
22422600.

19. Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, et al. 2012; Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 307:1169–77. DOI:
10.1001/jama.2012.316. PMID:
22436957.
20. Wan CD, Cheng R, Wang HB, Liu T. 2008; Immunomodulatory effects of mesenchymal stem cells derived from adipose tissues in a rat orthotopic liver transplantation model. Hepatobiliary Pancreat Dis Int. 7:29–33.
21. Baertschiger RM, Serre-Beinier V, Morel P, Bosco D, Peyrou M, Clément S, et al. 2009; Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 4:e6657. DOI:
10.1371/journal.pone.0006657. PMID:
19684854. PMCID:
PMC2722022.

22. Yu CH, Chen HL, Chen WT, Ni YH, Lin YL, Chang MH. 2004; Portal hemodynamic changes after hepatocyte transplantation in acute hepatic failure. J Biomed Sci. 11:756–63. DOI:
10.1007/BF02254360. PMID:
15591772.

23. Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S. 1996; Studies of liver repopulation using the dipeptidyl peptidase IV-deficient rat and other rodent recipients: cell size and structure relationships regulate capacity for increased transplanted hepatocyte mass in the liver lobule. Hepatology. 23:482–96. DOI:
10.1002/hep.510230313. PMID:
8617428.

24. Soleimani M, Nadri S. 2009; A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 4:102–6. DOI:
10.1038/nprot.2008.221. PMID:
19131962.

25. Yu CH, Chen HL, Chen YH, Chang MF, Chien CS, Chang MH. 2009; Impaired hepatocyte regeneration in acute severe hepatic injury enhances effective repopulation by transplanted hepatocytes. Cell Transplant. 18:1081–92. DOI:
10.3727/096368909X12483162196647. PMID:
19650970.

26. Schäfer R, Spohn G, Baer PC. 2016; Mesenchymal stem/stromal cells in regenerative medicine: can preconditioning strategies improve therapeutic efficacy? Transfus Med Hemother. 43:256–67. DOI:
10.1159/000447458. PMID:
27721701. PMCID:
PMC5040900.

27. Wang J, Ren H, Yuan X, Ma H, Shi X, Ding Y. 2018; Interleukin-10 secreted by mesenchymal stem cells attenuates acute liver failure through inhibiting pyroptosis. Hepatol Res. 48:E194–202. DOI:
10.1111/hepr.12969. PMID:
28833919.

28. Lee SC, Kim KH, Kim OH, Lee SK, Hong HE, Won SS, et al. 2017; Determination of optimized oxygen partial pressure to maximize the liver regenerative potential of the secretome obtained from adipose-derived stem cells. Stem Cell Res Ther. 8:181. DOI:
10.1186/s13287-017-0635-x. PMID:
28774345. PMCID:
PMC5543744.

29. Possamai LA, Thursz MR, Wendon JA, Antoniades CG. 2014; Modulation of monocyte/macrophage function: a therapeutic strategy in the treatment of acute liver failure. J Hepatol. 61:439–45. DOI:
10.1016/j.jhep.2014.03.031. PMID:
24703954.

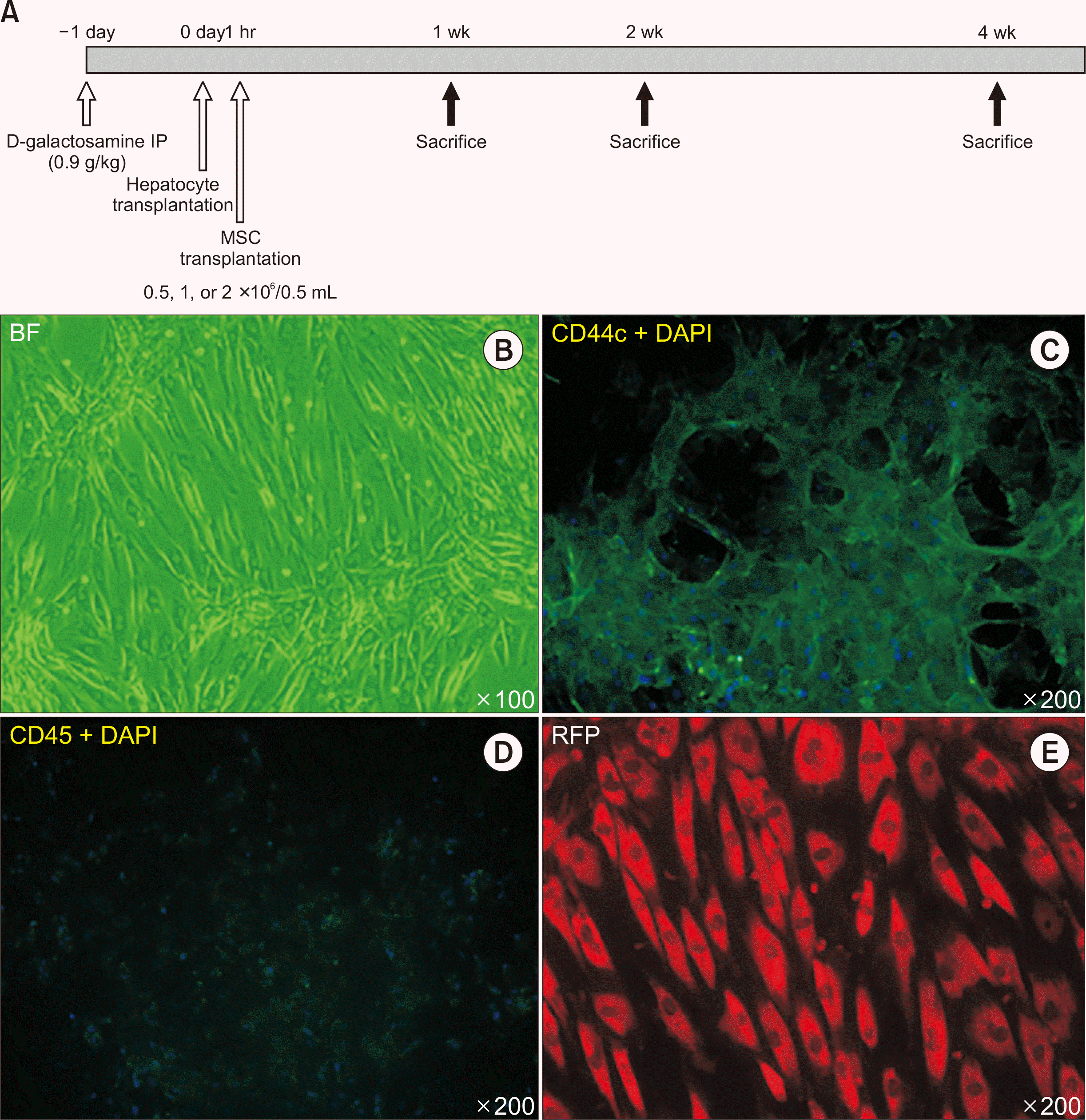
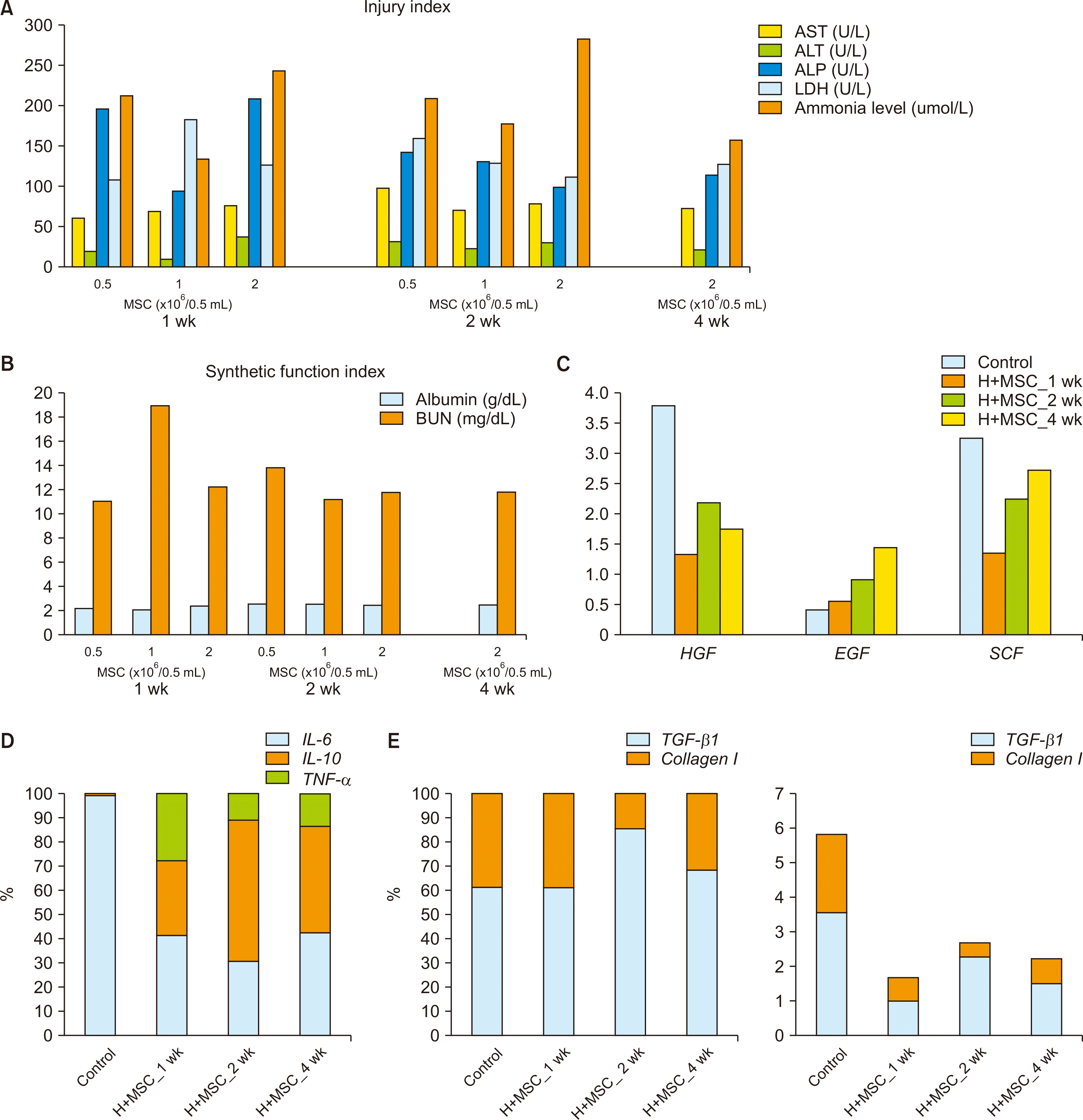




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download