Abstract
Graft-versus-host disease (GVHD) is rare complication after simultaneous pancreas-kidney transplantation (SPK). Here, we present a rare case of GVHD that developed in a SPK recipient. A 38-year-old female patient with end-stage renal disease due to type 1 diabetes mellitus underwent SPK from a deceased female donor. Four months after transplantation, she was readmitted with fever and abdominal pain and computed tomography revealed a retroperitoneal abscess. While being treated for the retroperitoneal abscess by percutaneous drainage and antibiotics, the patient developed skin rash and diarrhea. A skin biopsy performed at the time showed vacuolization and peri-venular lymphocytic infiltration compatible with GVHD. High-dose steroids resulted in complete remission of GVHD, and the patient was discharged after 2 weeks of treatment. This case demonstrates that early diagnosis of GVHD followed by timely treatment may led to a favorable outcome, and cautions that GVHD can occur in SPK patients.
Graft-versus-host disease (GVHD) is a multisystem disease that can occur after hematopoietic stem cell or solid organ transplantation [1,2]. It may occur when donor-derived T lymphocytes (graft) recognize recipient (host) as foreign and initiate hostile immune reaction against the recipient [3]. The organs targeted are skin, gastrointestinal tract, liver, and bone marrow, and these activities resulted in fever, skin rash, diarrhea, jaundice, and pancytopenia [4].
GVHD after solid organ transplant is rare because solid organs contain less lymphoid tissue than a hematopoietic stem cell transplant. In fact, only seven cases of GVHD following simultaneous pancreas-kidney transplantation (SPK) have been reported to date [1,4-9]. Nevertheless, GVHD after solid organ transplant is frequently lethal. Here, we report a successfully treated case of GVHD in an SPK recipient.
The study procedures were in accordance with the Declaration of Helsinki. All procedures were performed after obtaining informed consent. A single patient case report does not require Institutional Review Board approval according to our board policy.
The patient was a 38-year-old female with a 25-year history of type 1 diabetes mellitus who had been on hemodialysis for 7 years prior to SPK. She underwent SPK with organs from an ABO compatible, five (1A, 2B, 2DR) human leukocyte antigen-mismatched female donor. Both donor and recipient were positive for cytomegalovirus immunoglobulin G antibody. Induction immunosuppression included methylprednisolone and basiliximab followed by maintenance immunosuppression with tacrolimus, prednisone, and mycophenolate mofetil. Her postoperative course was complicated by duodenal bleeding, intra-abdominal fluid collection, and splenic vein (transplant pancreas) partial thrombus, which was treated with open thrombectomy per exploratory laparotomy.
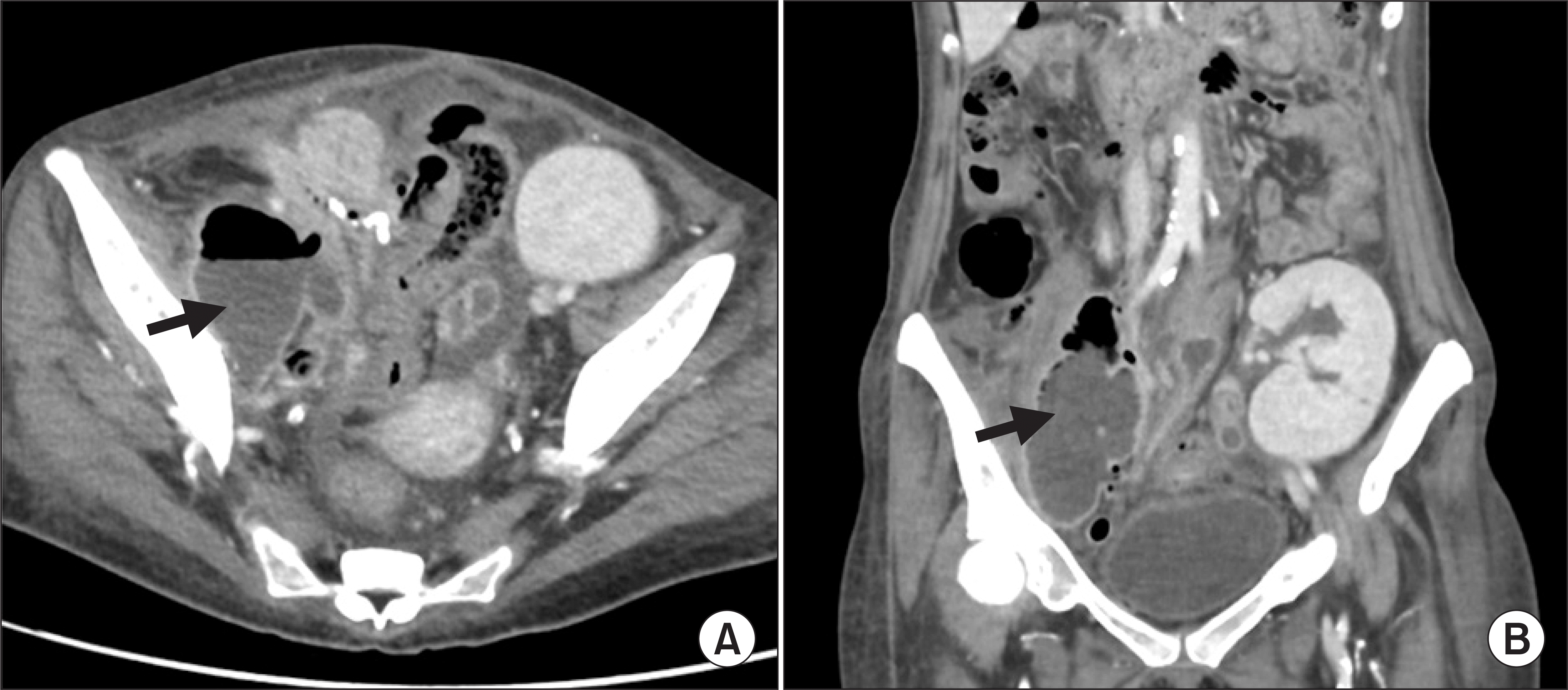
On postoperative day (POD) 114, she was readmitted with lower abdominal pain and fever. An abdomen and pelvis computed tomography depicted a retroperitoneal abscess in right pelvic area (Fig. 1). She underwent ultrasound-guided percutaneous catheter drainage and the culture showed extended spectrum β lactamase-producing Klebsiella pneumoniae and vancomycin-sensitive Enterococcus faecium. We started antibiotic treatment (imipenem+vancomycin) according to the results of culture and susceptibility tests. Mycophenolate mofetil was placed on hold for infection control. However, despite the above-mentioned treatment, a fistula developed between the retroperitoneal abscess and small bowel.
Six weeks later (POD 156), she developed erythematous macules on her trunk and fever (Fig. 2). Laboratory data showed a white blood cell count of 2,630 cells/mm3 (neutrophil, 74.2%; eosinophil, 0.7%; and lymphocyte, 16.6%), hemoglobin of 10.5 g/dL, and platelet count of 260,000/mm3. Serum creatinine, glucose, and liver function tests remained normal. Blood cytomegalovirus polymerase chain reaction was negative. She maintained tacrolimus at a dose of 0.1 mg/kg/day (blood trough level, 4 to 6 ng/mL) and prednisolone 5 mg/day. All antibiotics were withdrawn to rule out a drug reaction. Four days after the skin lesion presentation, skin biopsy was performed and it showed focal basal vacuolization and superficial peri-venular lymphocytic infiltration without hyperkeratosis, hypergranulosis, and acantosis, compatible with GVHD grade I. The patient was treated with intravenous methylprednisolone (500 mg) for 5 days, and given concerns of septic complications, was started on broad-spectrum antibiotics (cefepime, ciprofloxacin, and metronidazole). Tacrolimus dose was adjusted for the target blood trough level of 7 to 10 ng/mL. Mycophenolate mofetil was held given retroperitoneal abscess and neutropenia. The patient responded well to the treatment and was discharged 2 weeks later (POD 179) with stable graft function (creatinine 1.3 mg/dL and normoglycemia).
GVHD is rare but frequently fatal complication after solid organ transplantation, and the risk of its development is related to the number of donor lymphocytes transplanted with an organ [2]. Thus, because liver and multi-visceral transplants contain larger numbers of lymphocytes, the incidences of GVHD after these solid organ transplants are corresponding higher [10,11]. However, GVHD following SPK has rarely been reported. A review of the literature revealed GVHD after SPK has only been reported on seven occasions (Table 1), and that only two of the seven patients survived [1,4-9]. Here, we report a successfully treated case of GVHD after SPK.
Clinical manifestations of GVHD vary from skin rash to systemic involvement, including liver, gastrointestinal tract, and bone marrow. The common clinical manifestations of diarrhea and rash occur when donor-derived T lymphocytes are activated by alloantigens presented by host antigen-presenting cells and result in inflammatory response against host organs. Pancytopenia resulting from donor immune-mediated attack on recipient bone marrow is also a common feature [3,4].
Clinical features of GVHD are nonspecific and the diagnosis is often delayed because of similarities with drug reactions and infectious complications [12]. For example, ambiguous skin rash and fever may resemble a drug reaction or viral infection and diarrhea is also a common consequence of antibiotic treatment. In addition, the rarity of GVHD following SPK leads to delayed diagnosis, which contributes to the high mortality rate of GVHD. Our patient presented with many typical features, but the differential diagnosis was difficult because she was being treated with antibiotics. However, skin rash and diarrhea persisted after antibiotic withdrawal, and a skin biopsy performed 4 days after antibiotic withdrawal revealed focal basal vacuolization and perivenular lymphocytic infiltration. The patient received anti-GVHD treatment based on typical clinical manifestations and histologic confirmation of GVHD although chimerism study was not conducted. While fluorescence in situ hybridization can be helpful to detect chimerism in sex-mismatched cases, its use was limited in this sex-matched case.
The optimal treatment of GVHD after solid organ transplantation remains unclear. Given the mechanism of GVHD, which donor-derived T lymphocytes recognize recipient as foreign, inadequate immunosuppression might increase the risk of GVHD. Hence, the literature describes treatments that either enhance and reduce immunosuppression [2,7]. Some patients were treated by transplant pancreatectomy early in the course of GVHD, but clinical courses were not improved [1,9]. Recent reports on the topic have described successful treatment with high dose corticosteroid, as occurred in our patient [4,8]. Based on our experience of this case, early diagnosis of GVHD followed by timely treatment with sufficient immunosuppression appears to be the only way of achieving favorable outcomes.
In conclusion, transplant physicians should be aware GVHD can occur after SPK and that a high index of suspicion is essential to achieve early diagnosis as presenting signs and symptoms are nonspecific and can be mistaken for drug reactions or infections. Early diagnosis may allow successful treatment with supportive care aimed at preventing infections and multi organ failure.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding/Support
This study was supported by research grant from the Korean Society for Transplantation (2020-01-02001-010).
Author Contributions
Conceptualization: SIK. Data curation: JL, SIK. Formal analysis: JL. Funding acquisition: SIK. Methodology: JL, MSK, KHH, DJJ, JGL. Project administration: MSK, KHH, DJJ, JGL, SIK. Visualization: JL. Writing–original draft: JL. Writing–review & editing: JL, SIK.
REFERENCES
Gulbahce HE., Brown CA., Wick M., Segall M., Jessurun J. 2003. Graft-vs-host disease after solid organ transplant. Am J Clin Pathol. 119:568–73. DOI: 10.1309/395BX683QFN6CJBC. PMID: 12710129.

Zhang Y., Ruiz P. 2010. Solid organ transplant-associated acute graft-versus-host disease. Arch Pathol Lab Med. 134:1220–4. DOI: 10.1111/ctr.12797. PMID: 20670147.

Shin CR., Nathan J., Alonso M., Yazigi N., Kocoshis S., Tiao G, et al. 2011. Incidence of acute and chronic graft-versus-host disease and donor T-cell chimerism after small bowel or combined organ transplantation. J Pediatr Surg. 46:1732–8. DOI: 10.1016/j.jpedsurg.2011.04.016. PMID: 21929982.

Chang Jw., Sageshima J., Ciancio G., Mattiazzi A., Chen L., Tsai HL, et al. 2014. Successful treatment for graft-versus-host disease after pancreas transplantation. Clin Transplant. 28:217–22. DOI: 10.1111/ctr.12300. PMID: 24433450.

Kimball P., Ham J., Eisenberg M., King A., Fisher R., Rhodes C, et al. 1997. Lethal graft-versus-host disease after simultaneous kidney-pancreas transplantation. Transplantation. 63:1685–8. DOI: 10.1097/00007890-199706150-00025. PMID: 9197367.

Asari S., Matsumoto I., Toyama H., Shinzeki M., Goto T., Tanaka M, et al. 2015. Acute graft-versus-host disease following simultaneous pancreas-kidney transplantation: report of a case. Surg Today. 45:1567–71. DOI: 10.1007/s00595-014-1069-z. PMID: 25373363.

Osband AJ., Laskow DA., Mann RA. 2010. Treatment of acute graft-vs-host disease after simultaneous pancreas-kidney transplantation: a case report. Transplant Proc. 42:3894–7. DOI: 10.1016/j.transproceed.2010.08.058. PMID: 21094880.

Rossi AP., Bone BA., Edwards AR., Parker MK., Delos Santos RB., Hagopian J, et al. 2014. Graft-versus-host disease after simultaneous pancreas-kidney transplantation: a case report and review of the literature. Am J Transplant. 14:2651–6. DOI: 10.1111/ajt.12862. PMID: 25219902.

Weinstein A., Dexter D., KuKuruga DL., Philosophe B., Hess J., Klassen D. 2006. Acute graft-versus-host disease in pancreas transplantation: a comparison of two case presentations and a review of the literature. Transplantation. 82:127–31. DOI: 10.1097/01.tp.0000225832.47130.10. PMID: 16861952.

Ganoza A., Mazariegos GV., Khanna A. 2019. Current status of graft-versus-host disease after intestinal transplantation. Curr Opin Organ Transplant. 24:199–206. DOI: 10.1097/MOT.0000000000000624. PMID: 30762668.

Taylor AL., Gibbs P., Bradley JA. 2004. Acute graft versus host disease following liver transplantation: the enemy within. Am J Transplant. 4:466–74. DOI: 10.1111/j.1600-6143.2004.00406.x. PMID: 15023138.

Kim GY., Schmelkin LA., Davis MD., El-Azhary RA., Farrell AM., Meves A, et al. 2018. Dermatologic manifestations of solid organ transplantation-associated graft-versus-host disease: a systematic review. J Am Acad Dermatol. 78:1097–101. DOI: 10.1016/j.jaad.2017.12.050. PMID: 29288097. PMCID: PMC6167008.

Table 1
Treatment and outcomes of prior reported cases of GVHD following SPK
| Study | Age (yr) | Sex | Donor graft | HLA mismatch | Onset (POD, day) | Treatment | Outcome (recovery time) |
|---|---|---|---|---|---|---|---|
| Kimball et al. [5] | 27 | F | Cadaveric SPK | 5 | 9 | IS reduction, G-CSF | Death |
| Weinstein et al. [9] | 38 | M | Cadaveric pancreas, living kidney | 0 | 27 | IS reduction, graft pancreatectomy, lymphocyte immune globulin, cyclosporine, steroid | Death |
| 3 | |||||||
| Gulbahce et al. [1] | 58 | NA | Cadaveric SPK | 4 | NA | Graft removal | Death |
| Osband et al. [7] | 45 | M | Cadaveric SPK | 2 | 15 | ATG, steroid, increased IS | Death |
| Chang et al. [4] | 61 | M | Cadaveric SPK | NA | 43 | High dose steroid, topical IS | Recovery (2 wk) |
| Rossi et al. [8] | 29 | F | Cadaveric SPK | 3 | 12 | High dose steroid, increased IS | Recovery (5 wk) |
| Asari et al. [6] | 37 | M | Cadaveric SPK | 0 | 63 | High dose steroid | Death |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download