INTRODUCTION
Cytomegalovirus (CMV) infection is a crucial infection in kidney transplant recipients (KTRs) [
1]. CMV infection usually happens within 1 year after kidney transplantation (KT), which is an unstable period of immunosuppression and ultimately can lead to early graft failure [
1]. Recently, the prevalence of CMV infection is increasing due to the use of strong immunosuppressants such as antithymocyte globulin (ATG), rituximab, or bortezomib [
2]. If proper treatment is not provided in this condition, CMV that has invaded multiple organs can lead to death [
3]. In addition, bacterial infections, other viral infections, and fungal infections can often occur, and acute rejection can occur simultaneously [
4,
5]. Thus, it is important to detect and treat CMV infection early and prevent it, if possible.
Even though the diagnosis and treatment for CMV infection have been developed, many controversies exist about the ways to prevent CMV infection, and each center’s policies vary. The Kidney Disease: Improving Global Outcomes guidelines and the US guidelines recommended CMV prophylaxis in KTRs with a high risk of CMV infection, that is, kidney donors whose CMV serostatus was positive and recipients whose CMV serostatus was negative before KT, for 3 months after KT, and for 6 weeks after anti-rejection therapy such as steroid pulse therapy or the use of ATG [
3,
6-
8]. In our center, we used intravenous ganciclovir for 2 weeks for CMV prophylaxis during the recovery period after KT, but we could not decrease the incidence of CMV infection. Most guidelines recommended oral valganciclovir for CMV prophylaxis, but oral valganciclovir was very expensive and has some adverse effects. Recent research reported that the effect of oral valacyclovir is not inferior to that of oral valganciclovir [
9], but its efficacy remains unclear. Therefore, we evaluated the effectiveness of oral valacyclovir prophylaxis for 3 months in comparison with the use of an intravenous ganciclovir for 2 weeks to prevent CMV infection in KTRs.
Go to :

METHODS
This study was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center (IRB No. 2017-10-026). All clinical investigations were conducted in accordance with the guidelines of the 2013 Declaration of Helsinki. The clinical and imaging data were obtained with patients’ consent for the purpose of scientific research publication.
Study Design
We retrospectively investigated 153 KTRs between September 2013 and January 2016. In this study, we classified the participants into two groups as follows: valacyclovir prophylaxis group (intravenous ganciclovir for 2 weeks after KT, followed by oral valacyclovir for 3 months from July 2015) and historical control group (only intravenous ganciclovir for 2 weeks after KT from study starting time to June 2015), as seen in
Fig. 1. Blood CMV polymerase chain reaction (PCR) was checked at KT, at 1 month after KT, 3 months after KT, and once a month from 3 to 12 months after KT and once a year after 12 months after KT. Whole blood samples were tested by using QIAcube (Qiagen, Hilden, Germany) and real-time PCR assays for CMV. The result of CMV PCR was expressed to log
10 copies/mL. CMV infection is defined as virus isolation or detection of viral proteins or nucleic acid in any body fluid or tissue specimen [
10]. Several specific definitions for CMV detection in blood are recommended such as the detection of virus, antigen, or DNA. Therefore, CMV infection was diagnosed when the blood CMV PCR was more than 100 log
10 copies/mL as cutoff values of positive blood CMV PCR, irrespective of the symptoms. In addition, CMV disease was diagnosed as the CMV infection with accompanying symptoms. CMV disease could be classified as viral syndromes with fever, malaise, leukopenia, thrombocytopenia, or as a tissue invasive disease [
8]. We evaluated the incidence of CMV infection, clinical outcomes, and CMV-free survival rate between the two groups and risk factors for the development of CMV infection.
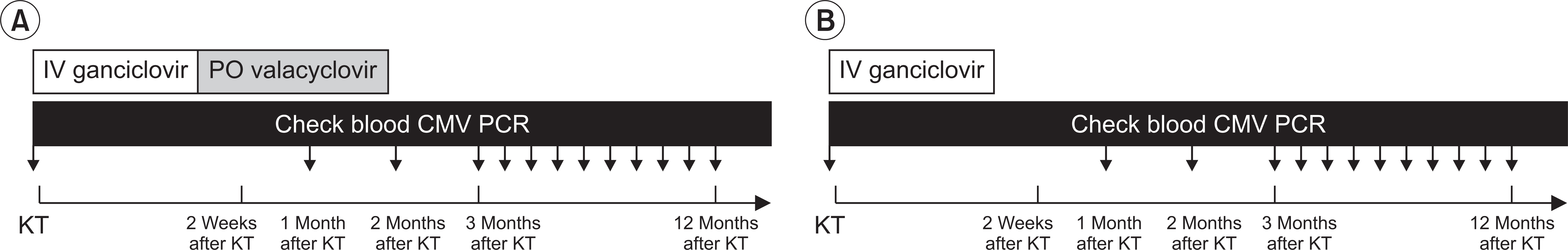 | Fig. 1Study protocol. We classified into the two groups as follows: valacyclovir prophylaxis group (IV ganciclovir for 2 weeks after KT, followed by oral valacyclovir for 3 months) (A) and control group (only intravenous ganciclovir for 2 weeks after KT) (B). KT, kidney transplantation; IV, intravenous; PO, per oral; CMV, cytomegalovirus; PCR, polymerase chain reaction. 
|
Immunosuppression Protocols
We administered basiliximab (20 mg at days 0 and 4, respectively; Simulect, Novartis, Basel, Switzerland) as the immunosuppressant for induction in KTRs with low immunologic risks. We also administered ATG (Thymoglobulin, Genzyme, Cambridge, MA, USA; 1.5 mg/kg at day 0 and 1.0 mg/kg between days 1 and 3) in KTRs with high immunologic risks such as 2nd KT, high panel reactive antibody, and fully mismatched human leukocyte antigen (HLA). We used tacrolimus (Prograf; Astellas Pharma Inc., Toyama, Japan), prednisolone, and mycophenolate mofetil (MMF; Cellcept, Hoffmann-La Roche Inc., Nutley, NJ, USA) as the immunosuppressive regimen for maintenance.
We maintained trough levels of tacrolimus at 5–10 ng/mL for 1 month after KT and at 3–8 ng/mL after that. Prednisolone (30 mg/day) was administered for 2 weeks and was reduced by 10 mg/day. MMF was administered at 1,000 mg/day beginning a month after KT.
In ABO-incompatible KT recipients and highly sensitized recipients, rituximab (200 and 375 mg/m2, respectively; Roche Pharma AG, Reinach, Switzerland) was administered once at 2 weeks before KT. Plasmapheresis with 5% albumin and fresh frozen plasma just before KT was performed, and administration of intravenous immunoglobulin (100 mg/kg) was performed every other day for desensitization.
Patients received intravenous ganciclovir (Cymevene, Roche Pharma, Basel, Switzerland; 2.5–5.0 mg/kg, twice a day according to estimated glomerular filtration rate [eGFR]) against CMV infection for 14 days after KT. Ganciclovir was administered intravenously in the valacyclovir prophylaxis group for 2 weeks after KT, followed by valacyclovir (Valtrex; Glaxo Wellcome, Dartford, UK; a total of 8.0 g, 2.0 g four times a day for normal renal function) orally for 3 months. The doses of antiviral drugs were tapered on the basis of renal function [
11]. All KTRs received trimethoprim-sulfamethoxazole (80 mg/400 mg, twice a day) against
Pneumocystis jiroveci pneumonia and oral fluconazole (5 mL, once a day) against fungal infection for 1 month.
Demographics of KTRs
We investigated donor and recipient age at KT, sex, donor type, the number of KT, dialysis type before KT, etiologies of end-stage renal disease, the number of HLA mismatches, immunosuppressant for induction and maintenance, the rate of biopsy-proven acute rejection, delayed graft function, panel reactive antibody >50%, positive donor specific antibody, CMV infection or disease, BK virus associated nephropathy, allograft function at diagnosis, the proportion of allograft failure, patient death, and time between KT and CMV infection.
Statistical Analysis
Student t-test was performed for continuous variables with a normal distribution, and the variables were expressed as the mean±standard deviation. Chi-square or Fisher’s exact test was performed for categorical variables, and the variables were expressed as the numbers and percentages. CMV-free graft survival rate was obtained by the Kaplan-Meier analysis with log-rank test. Risk factors for CMV infection were analyzed by logistic regression analysis. The P-values <0.05 were statistically significant. Statistical analysis was performed by SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Go to :

DISCUSSION
Prophylaxis for CMV infection after KT is very important because of early graft failure due to CMV infection [
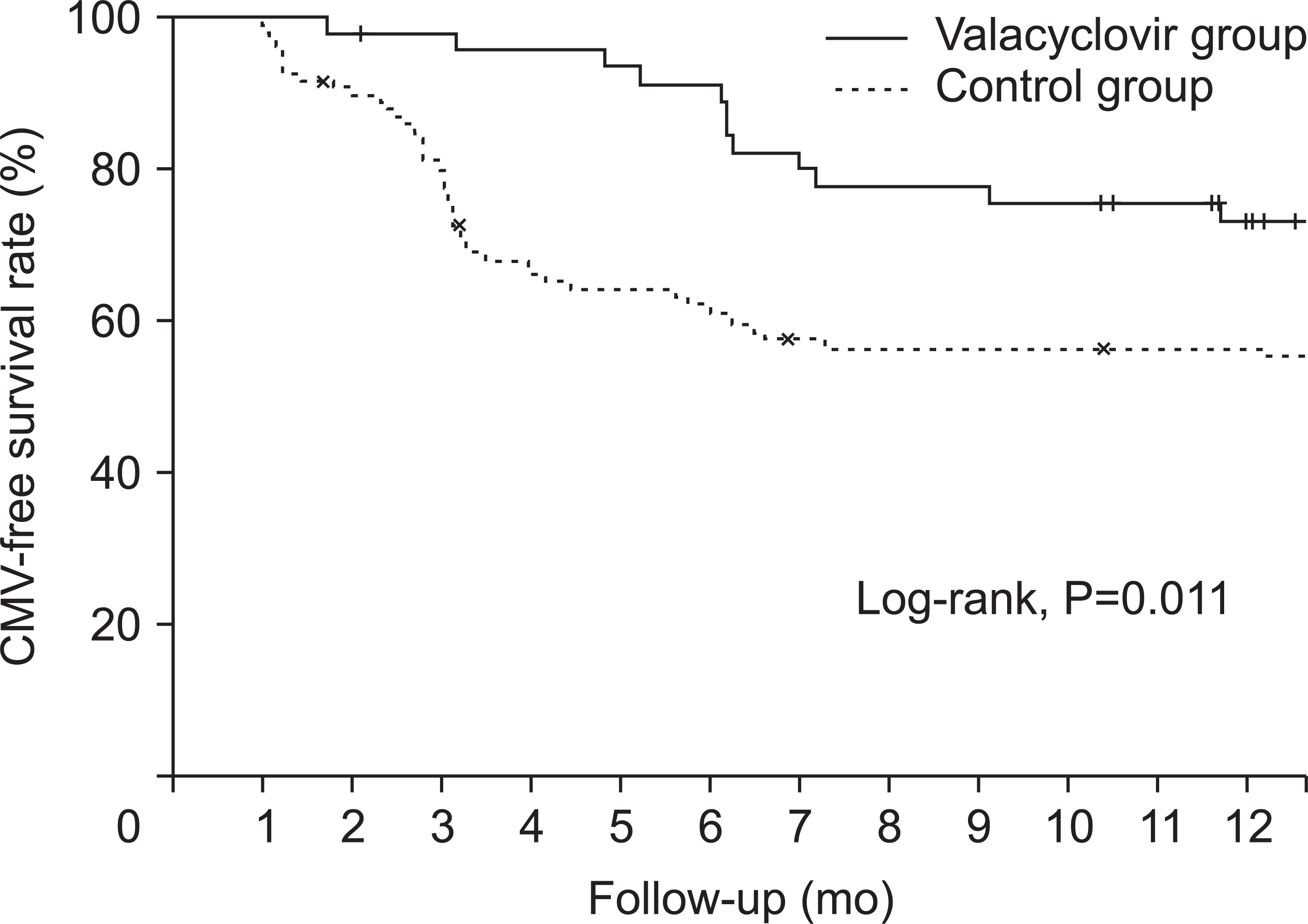
1]. We used intravenous ganciclovir to prevent CMV infection for only 2 weeks of hospitalization for postoperative care after KT since 2009, but we could not effectively prevent the occurrence of CMV infection. Therefore, we used oral valacyclovir for 3 months after intravenous administration of ganciclovir for 2 weeks from July 2015 to prevent CMV infection even if patients were at low risk for CMV infection. Our study compared the valacyclovir prophylaxis group with historical control group using the intravenous ganciclovir only from the beginning of the study to June 2015. Our study showed a significantly lower incidence of CMV infection in the valacyclovir prophylaxis group than in the control group.
Some guidelines recommend the use of oral valganciclovir (Valcyte; Hoffman-La Roche, Grenzach-Wyhlem, Germany) to prevent CMV infection [
12]. However, some research reported that the efficacy of oral valacyclovir was not inferior to that of oral valganciclovir in the prevention of CMV infection [
9]. A study reported that the incidence of polyomavirus viremia was higher in the valganciclovir group than in the oral valacyclovir group [
9]. Although we could not directly compare oral valacyclovir with oral valganciclovir in our study, we could predict that oral valacyclovir was also safe since there was no significant difference in the incidence of BK virus infection between the valacyclovir group and the control group (
Table 3). The incidence of biopsy-proven acute rejection was also very low in the valacyclovir group in our study, although the other research reported that the incidence of biopsy-proven acute rejection was higher in the valacyclovir prophylaxis group than in the valganciclovir prophylaxis group [
9]. In our study, leukopenia and thrombocytopenia occurred in the valacyclovir group, similar to other studies, but leukopenia and thrombocytopenia improved in all patients after dose reduction (
Table 3) [
9]. Oral valacyclovir is also more cost effective than oral valganciclovir [
13]. However, the number of tablets of valacyclovir was too many according to the allograft function in comparison with that of tablets of valganciclovir, and the compliance of taking medicine was a little low.
It is well-known that the risk of CMV infection is very high when ATG, as an immunosuppressive agent for induction, is used [
2]. Our study also showed that the proportion of CMV infection was significantly higher in the KTRs with ATG induction compared to the basiliximab induction in
Table 3 (P<0.001). However, the incidence of CMV infection tended to be lower in the valacyclovir prophylaxis group (40.0%) than in the control group (67.6%) in the KTRs with ATG induction as in other studies in
Fig. 2D [
14].
CMV infections can often be accompanied with acute rejection [
5]. In our study, the proportion of biopsy-proven acute rejection was higher in the CMV infection group compared to non-CMV infection group. However, the proportion of biopsy-proven acute rejection did not differ significantly between the valacyclovir prophylaxis and control groups. Therefore, valacyclovir prophylaxis might reduce the development of acute rejection accompanied with CMV infection.
CMV and BK virus infections can result in allograft loss in KTRs, but the interactions between the two infections are uncertain. The effectiveness of valacyclovir for BK virus infection is also uncertain. Our study showed the proportion of BK virus infection was significantly higher in the CMV infection group. Nevertheless, the incidence of BK virus infection tended to be lower in the valacyclovir prophylaxis group than in the control group in our study. CMV infection might indirectly protect against BK virus infection due to reduction of immunosuppressant dose after diagnosis of CMV infection, and not the effect of valacyclovir [
15]. Further studies should be conducted to understand the association between CMV and BK virus infections.
In our study, we controlled the dosage of valacyclovir according to the eGFR. The dosage was varied in different centers. A study showed that the prognosis of low dose valacyclovir (3.0 g/day) was as effective as the standard dose [
16], and in our study, we used a higher dose of valacyclovir (6.0 g/day), and the results of the dosage of valacyclovir in our study was not inferior to that of low dose and had fewer side effects. The main complication of valacyclovir is neurotoxicity due to high dose such as 8.0 g/day, but in our study, there were no significant neurologic symptoms such as hallucinations or confusion [
16]. Only the patients using the maximum prescribed dosage of valacyclovir according to the eGFR had leukopenia (9, 19.6%) and thrombocytopenia (5, 10.9%) (
Table 2). After we reduced the dosage of valacyclovir, the complications resolved completely.
Our study has some limitations. First, our study was a retrospective, single-center study; thus, selection bias could not be avoided. Second, the sample size was small. Third, CMV serostatus of all donors and recipients was positive. Finally, even though the occurrence of CMV infection could be reduced after valacyclovir prophylaxis, the incidence of CMV infection was still high. In conclusion, valacyclovir prophylaxis effectively decreased the occurrence of CMV infection in KTRs in our study. Therefore, we should use valacyclovir prophylaxis for 3 months in KTRs with risk factors such as old age, thymoglobulin induction, and delayed graft function.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download