Abstract
Background
Renal transplant is an effective treatment option for end-stage kidney disease and tacrolimus is one of the most commonly used immunosuppressant drugs in renal transplant patients. Tacrolimus is a substrate of cytochrome P450 3A5 (CYP3A5), and a narrow therapeutic index and large inter-individual variability. The objective of this study was to determine the frequency of CYP3A5*3 polymorphism and its effect on the pharmacokinetics of tacrolimus in post-renal transplant patients at Mandalay General Hospital.
Methods
Three different genotypes of CYP3A5 were determined by polymerase chain reaction-restriction fragment length polymorphism in 54 post-renal transplant recipients. Tacrolimus trough concentrations (Ctrough) were measured by enzyme multiplied immunoassay technique following method validation. The apparent clearance (CL/F) was calculated from measured Ctrough.
Results
The frequency of CYP3A5*1 allele was 0.24 and that of CYP3A5*3 allele was 0.76 in the study sample. There were a total of 33 CYP3A5 non-expressors (patients with CYP3A5*3/*3 genotype) and 21 CYP3A5 expressors (patients with CYP3A5*1/*1 or CYP3A5*1/*3 genotype), respectively. CL/F was significantly lower in the non-expressor group than in the expressor group (mean±standard deviation, 12.53±5.28 vs. 21.22±5.95 L/hr; P<0.001). The mean Ctrough and concentration dose ratio (C/D) for tacrolimus in CYP3A5 non-expressors were significantly higher than those in CYP3A5 expressors (Ctrough, 7.14±2.56 vs. 5.92±1.76 ng/mL; C/D, 6.92±4.11 vs. 2.89±1.85 ng/mL/mg), respectively (P<0.05).
Conclusions
In this study, 76% of the Myanmar renal transplant recipients carried the CYP3A5*3 allele that was associated with lower functional activity of the CYP3A5 enzyme a lower CL/F of tacrolimus in these Thus, CYP3A5 polymorphism influences the pharmacokinetics of tacrolimus, which may affect the pharmacological response to tacrolimus
For patients with end-stage kidney disease, kidney transplantation is a successful means of improving their quality of life. Immunosuppressive agents are usually taken lifelong by kidney transplant patients since it is critical to prevent episodes of both, early and late acute rejection, as well as chronic allograft nephropathy in transplant patients [1]. Tacrolimus is one of the most commonly used immunosuppressant drugs in renal transplant patients. A successful transplantation outcome depends on an optimal balance between under-immunosuppression and over-immunosuppression [2]. Tacrolimus is used as the backbone of immunosuppressant therapy for more than 90% of all renal transplant recipients in the United States [3]. In Thailand, 83% of all renal transplant recipients used tacrolimus as part of their immunosuppressant regimen [4].
As a substrate of cytochrome P450 3A5 (CYP3A5), tacrolimus is characterized by a narrow therapeutic index and a large inter-individual pharmacokinetic variability. Several factors influence the pharmacokinetics of tacrolimus, including hematocrit, serum albumin, ethnicity, drug interactions, as well as genetic polymorphisms [5]. CYP3A5 genetic polymorphisms may be associated with variation in the pharmacokinetics of tacrolimus in Asian populations. It is one of the main enzymes that metabolizes tacrolimus, and its genetic polymorphism is common among Asian populations. CYP3A4 is also involved in the metabolism of tacrolimus, however, CYP3A4 polymorphisms are probably rare in Asians populations [6-11].
CYP3A5*3 is the most common non-functional variant of CYP3A5, while CYP3A5*1 is the functional CYP3A5 variant [10]. Individuals with the CYP3A5*3/*3 genotype are termed as CYP3A5 non-expressors while those with the CYP3A5*1/*1 and CYP3A5*1/*3 genotypes are termed as CYP3A5 expressors [12]. The allele frequency of CYP3A5*3 among South Asian and East Asian populations is 66.8% and 71.3% [13].
In a previous study in 173 post-renal transplant patients, CYP3A5 expressors exhibited a higher apparent oral tacrolimus clearance than CYP3A5 non-expressors [14]. However, Boudia et al. [15] reported that the CYP3A5 polymorphisms did not influence tacrolimus pharmaco kinetics in an Algerian population (n=62). Further, tacrolimus clearance was not related to the CYP3A5 genotype in stable renal transplant recipients (n=118) [16].
Thus immunosuppression with tacrolimus is still unsuccessful in some patients, while causing toxicity in others. CYP3A5 polymorphism is associated with variation in tacrolimus pharmacokinetics in many ethnic groups. However, limited data is available in Myanmar renal transplant recipients about the effects of CYP3A5 polymorphism on the pharmacokinetics of tacrolimus. Therefore, this study aimed to determine the frequency of the CYP3A5*3 genetic polymorphism and its effect on the pharmacokinetics of tacrolimus in renal transplant recipients.
Descriptive study was conducted for genotype frequency of CYP3A5*3/*3, CYP3A5*1/*1, and CYP3A5*1/*3 and hospital based cross-sectional comparative study was conducted for pharmacokinetic study of tacrolimus in those genotype groups. This study was conducted after ethical approval by the Research Ethics Committee of the University of Medicine, Mandalay (ID No. 23 [pharmaco] UMM/2016). Written informed consent was obtained from all patients. Fifty-four stable renal transplant recipients (Myanmar Ethnic) who underwent renal transplantation at Mandalay General Hospital were included this study.
The study was conducted at the Common Research Laboratory and Department of Pharmacology at the University of Medicine Mandalay. There were total 76 numbers of post renal transplant patients. All renal transplant recipients (1 to 12 months posttransplant) from Mandalay General Hospital that were receiving tacrolimus Pangraf (Panacea Biotec, New Delhi, India), prednisolone, and mycophenolate mofetil as immunosuppressant agents, were invited to participate in this research. Patients who were prescribed cyclosporine were excluded from this study. The objective and procedures of the study were explained in detail to each patient, and thereafter their decision to participate in the study was entirely voluntary. Tacrolimus doses were administered twice daily and adjusted to trough concentrations (Ctrough) between 5 and 12 ng/mL using aminimization dosing protocol, posttransplant duration, and clinical response. A whole blood sample (5 mL) was collected (15–30 minutes) prior to administration of the immunosuppressive drug and stored at –20°C until analysis.
All patient samples were analyzed in the Common Research Laboratory (University of Medicine Mandalay) using polymerase chain reaction (PCR)-restriction fragment length polymorphism analysis. Purified genomic DNA was extracted by Geneaid DNA extraction kit (Geneaid Biotech Ltd; Shijr City, New Taipei City, Taiwan) from 200 µL of the whole blood samples (collected in ethylenediaminetetraacetic acid tubes) following manufacturer instructions. The extracted DNA was stored at –20°C until analysis. PCR amplification and digestion with specific restriction enzyme DdeI (New England Biolabs, Ipswich, MA, USA) were performed as per, previously described methods [17].
Tacrolimus whole blood Ctrough were measured using Enzyme Multiplied Immunoassay Technique (EMIT; Siemens Healthcare Diagnostics, Newark, DE, USA) after method validation. Assay validation was conducted with a calibration standard curve ranging from 0 to 30 ng/mL. The linearity of the assay method was evaluated using six different concentrations of tacrolimus (0, 2.5, 5, 10, 20, and 30 ng/mL). Three quality control (QC) samples were prepared at 4.6, 11.0, and 22.0 ng/mL (Siemens) and were coanalyzed with all samples and standards. The inter-day and intra-day coefficient of variation for each QC sample was <4%, and <8%, respectively.
The apparent clearance (CL/F) was calculated from measured tacrolimus Ctrough by JPKD version 3.0 software (Pharmdatatech, Taiwan). The software is the CL/F program based on Bayesian pharmacokinetic estimation models using published population pharmacokinetic parameters and is easy to use. The parameters, including tacrolimus Ctrough, tacrolimus concentration dose ratio (C/D) and CL/F were compared between CYP3A5 non-expressor and expressor groups.
The data were collected with proforma. After checking for completeness, the data were analyzed by Stata ver. 13 (Stata Corp., College Station, TX, USA). The frequency of the different genotypes (CYP3A5*3/*3, CYP3A5*1/*1, and CYP3A5*1/*3) were estimated by counting and expressed as a percentage. Allelic (CYP3A5*1 and CYP3A5*3) frequencies were calculated using a Hardy-Weinberg equilibrium calculator [18]. Mann-Whitney U-tests were used for comparison of tacrolimus Ctrough, C/D, and CL/F between the non-expressors (CYP3A5*3/*3) and expressor (CYP3A5*1/*1 and CYP3A5*1/*3) groups. A P-value <0.05 was considered as statistically significant.
The allelic frequency for CYP3A5 was calculated using the Hardy-Weinberg equilibrium calculator. The observed frequencies of CYP3A5*1 and CYP3A5*3 were 0.24 and 0.76, respectively (Fig. 1), which were not different from their expected frequencies. The characteristics of the renal transplant patients (Myanmar ethnics, n=54) are described in Table 1.
The means and standard deviations (SDs) of the measured whole blood trough C/D and CL/F of tacrolimus in CYP3A5 non-expressor and CYP3A5 expressor groups are shown in Fig. 2. The CL/F of tacrolimus in CYP3A5 non-expressors was significantly lower than that in CYP3A5 expressors (P<0.05).
In this study, the frequency of the CYP3A5*3 polymorphism was identified in 54 post-renal transplant patients (Myanmar ethnics) with post-renal transplant duration of 1 to 12 months were recruited in order to include patients in a stable posttransplant condition. The pharmacokinetics of tacrolimus were compared between the CYP3A5 non-expressor (CYP3A5*3/*3) and CYP3A5 expressor (CYP3A5*1/*1 and CYP3A5*1/*3) genotype groups.
The mean±SD whole blood Ctrough of tacrolimus in the CYP3A5 non-expressor and expressor groups were 7.14±2.56 and 5.92±1.76 ng/mL, respectively. These were within the target therapeutic range of 5 to 20 ng/mL. The mean Ctrough of the CYP3A5 non-expressor group was significantly higher than that of the CYP3A5 expressor group (P<0.001). Tacrolimus is primarily metabolized by the highly polymorphic CYP3A5 enzyme in the liver.
The CYP3A5*3 non-expressor group had higher trough tacrolimus concentrations than the CYP3A5*3 expressor group, suggesting that tacrolimus metabolism in patients with the CYP3A5*3/*3 genotype is higher than that in patients with CYP3A5*1/*3 and CYP3A5*1/*1 genotypes. These results are similar to those found in a study conducted in 165 renal transplant patients, where the tacrolimus Ctrough in the CYP3A5 non-expressor group were significantly higher than those of the CYP3A5 expressor group (P<0.05), suggesting that CYP3A5*3 is a major genetic factor affecting tacrolimus concentrations in renal transplant recipients [19]. Additionally, in studies evaluating Korean patients carrying the CYP3A5*3 allele, homozygotes showed significantly higher tacrolimus concentrations than patients carrying at least one CYP3A5*1 allele (n=12), and CYP3A5 polymorphism significantly affected the achievement of target tacrolimus trough levels (n=62) [20,21].
The mean C/D of tacrolimus for the CYP3A5 non-expressor group (6.92±4.11 ng/mL/mg) was significantly higher than that for the CYP3A5 expressor group (2.89±1.85 ng/mL/mg, P<0.001). This finding was also reported in other studies [22-26], suggesting that the CYP3A5 6986A>G polymorphism in renal transplant recipients influences the tacrolimus C/D. The mean CL/F in the CYP3A5 non-expressor group (12.53±5.28 L/hr) was significantly lower than that in the CYP3A5 expressor group (21.22±5.95 L/hr, P<0.001). In a previous study the pharmacokinetics of tacrolimus, CL/F was lower in the CYP3A5 non-expressor group than in the CYP3A5 expressor group [12,27].
Tacrolimus is considered as a backbone agent in immunosuppression protocols for the management of renal transplant patients, and the drug dose is adjusted according to routine monitoring of its blood levels in order to maintain adequate immunosuppression to avoid acute rejection and to minimize adverse effects. Other variables that may contribute to the pharmacokinetic differences between groups were investigated by comparing demographic factors including age, sex, and body weight between the and non-expressor groups. No demographic factor showed a significant difference between the two groups other than sex. However, Fitzsimmons et al. [28] observed no sex-specific effects on the pharmacokinetic profile of tacrolimus. Therefore, variations in whole blood tacrolimus concentration in this study are likely to be related to the genetic polymorphism of CYP3A5*3.
Factors other than CYP3A5 polymorphism that can affect the CL/F of tacrolimus include age, race, posttransplant duration, albumin levels, hematocrit levels, and corticosteroid dose. A lower hematocrit and a lower plasma albumin concentration may result in a higher unbound fraction of tacrolimus and a consequent increase in clearance. Conversely, a higher hematocrit and a higher plasma albumin concentration would result in a lower clearance of tacrolimus [29]. However, in the current study, posttransplant duration and plasma albumin levels in all patients were comparable and not different between the CYP3A5 expressor and non-expressor groups. Corticosteroid dose in all patients were also comparable and not different between these two groups. The assay used in this study, the EMIT 2000 tacrolimus assay, was reported to be free from hematocrit interference in the range of 25%–60%, and all the patients in this study had hematocrit levels in the range of 25%–55%. Therefore, the possibility of a bias for low or high hematocrit values to tacrolimus clearance can be ruled out.
In this study, there were significant differences in tacrolimus Ctrough, C/D, and CL/F between CYP3A5 non-expressor and expressor groups. Clearance is one of the most important parameters in clinical pharmacokinetics. Clearance may be large, if enzymes have a high capacity to metabolize a drug, and fit may be small if enzymes have a limited capacity to metabolize the drug [30]. Thus, a significantly lower CL/F of tacrolimus in the non-expressor group may signify that individuals with two alleles of CYP3A5*3 (CYP3A5*3/*3 mutant homozygous genotype) cannot express the CYP3A5 protein adequately, and as a consequence, may have poor or no metabolizing activity. The wild-type CYP3A5*1 allele is associated with a greater production of the functional CYP3A5 enzyme, leading to higher drug metabolizing activity by CYP3A overall and a higher tacrolimus clearance.
Thus, based on the findings from this study, CYP3A5*3 polymorphism influences the pharmacokinetics of tacrolimus due to decreased functional activity of the enzyme that results in a decreased clearance of tacrolimus and a corresponding increase in tacrolimus blood concentration levels. This polymorphism may be a potential factor that contributes to the inter-individual variability in tacrolimus disposition in renal transplant patients in Myanmar. However, there was no significant effect on clinical outcomes of both groups because they all were alive and had stable renal function during the study period. Genotyping of patients for CYP3A5 polymorphism may support the prediction of an optimal starting and maintenance dose of tacrolimus and aid in identifying patients with an increased risk for drug inefficacy or side effects.
In this study, 76% of Myanmar renal transplant recipients carried the CYP3A5*3 allele. CYP3A5 enzyme activity and CL/F of tacrolimus was lower in these patients than in those carrying the CYP3A5*1 allele. Thus, CYP3A5 polymorphism may influence the pharmacokinetics of tacrolimus in the Myanmar population and consequently an effect on the pharmacological response to tacrolimus. Dosage adjustment of tacrolimus for personalized medicine is important to optimize efficacy, minimize toxicity, and improve the outcome of renal transplantation. There was a limited sample size in this study performed at the Mandalay General Hospital. Other genetic polymorphisms that may influence the pharmacokinetics of tacrolimus were not analyzed in this study due to financial limitation.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: all authors. Formal analysis, Data curation & Methodology: YYH. Project administration & Visualization: all authors. Writing–original draft: YYH. Writing–review & editing: all authors.
Additional Contributions
We thank our colleagues in Mandalay University of Medicine, Mandalay, Magway, and Mandalay General Hospital in management and staff for their support. We also thank our renal transplant team, laboratory team, and all participants.
REFERENCES
Patel DD., Modi KP., Patel AK. 2015. Prescription trends of immunosuppressant drugs in Indian renal transplant patients. Int J Pharm Sci Drug Res. 7:334–9.
Staatz CE., Goodman LK., Tett SE. 2010. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet. 49:141–75. DOI: 10.2165/11317350-000000000-00000. PMID: 20170205.

Chen L., Prasad GVR. 2018. CYP3A5 polymorphisms in renal transplant recipients: influence on tacrolimus treatment. Pharmgenomics Pers Med. 11:23–33. DOI: 10.2147/PGPM.S107710. PMID: 29563827. PMCID: PMC5846312.

Chan-On C., Sarwal MM. 2017. A comprehensive analysis of the current status and unmet needs in kidney transplantation in Southeast Asia. Front Med (Lausanne). 4:84. DOI: 10.3389/fmed.2017.00084. PMID: 28691007. PMCID: PMC5481314.

Shi WL., Tang HL., Zhai SD. 2015. Effects of the CYP3A4*1B genetic polymorphism on the pharmacokinetics of tacrolimus in adult renal transplant recipients: a meta-analysis. PLoS One. 10:e0127995. DOI: 10.1371/journal.pone.0127995. PMID: 26039043. PMCID: PMC4454552.

Hu YF., He J., Chen GL., Wang D., Liu ZQ., Zhang C, et al. 2005. CYP3A5*3 and CYP3A4*18 single nucleotide polymorphisms in a Chinese population. Clin Chim Acta. 353:187–92. DOI: 10.1016/j.cccn.2004.11.005. PMID: 15698606.

Hustert E., Haberl M., Burk O., Wolbold R., He YQ., Klein K, et al. 2001. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics. 11:773–9. DOI: 10.1097/00008571-200112000-00005. PMID: 11740341.

Kuehl P., Zhang J., Lin Y., Lamba J., Assem M., Schuetz J, et al. 2001. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 27:383–91. DOI: 10.1038/86882. PMID: 11279519.

Provenzani A., Santeusanio A., Mathis E., Notarbartolo M., Labbozzetta M., Poma P, et al. 2013. Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J Gastroenterol. 19:9156–73. DOI: 10.3748/wjg.v19.i48.9156. PMID: 24409044. PMCID: PMC3882390.

Lamba J., Hebert JM., Schuetz EG., Klein TE., Altman RB. 2012. PharmGKB summary: very important pharmacogene information for CYP3A5. Pharmacogenet Genomics. 22:555–8. DOI: 10.1097/FPC.0b013e328351d47f. PMID: 22407409. PMCID: PMC3738061.
Jada SR., Xiang X., Zhou Q., Li HH., Ooi LL., Chowbay B. 2006. Hepatic expression of CYP3A4 and CYP3A5 genes in Asians and implications for pharmacokinetic variations during chemotherapy. J Clin Oncol. 24(18 Suppl):13124. DOI: 10.1200/jco.2006.24.18_suppl.13124.
Barry A., Levine M. 2010. A systematic review of the effect of CYP3A5 genotype on the apparent oral clearance of tacrolimus in renal transplant recipients. Ther Drug Monit. 32:708–14. DOI: 10.1097/FTD.0b013e3181f3c063. PMID: 20864901.

Zhou Y., Ingelman-Sundberg M., Lauschke VM. 2017. Worldwide distribution of cytochrome P450 alleles: a meta-analysis of population-scale sequencing projects. Clin Pharmacol Ther. 102:688–700. DOI: 10.1002/cpt.690. PMID: 28378927. PMCID: PMC5600063.

Bergmann TK., Hennig S., Barraclough KA., Isbel NM., Staatz CE. 2014. Population pharmacokinetics of tacrolimus in adult kidney transplant patients: impact of CYP3A5 genotype on starting dose. Ther Drug Monit. 36:62–70. DOI: 10.1097/FTD.0b013e31829f1ab8. PMID: 24089074.
Boudia F., Boughrara W., Meriem A., Moghtit FZ., Hamdani A., Fetati H, et al. 2016. Effects of CYP3A5 and ABCB1 genetics variant on tacrolimus pharmacokinetics in Algerian adult renal transplant patients. J Med Sci Clin Res. 04:10182–8. DOI: 10.18535/jmscr/v4i4.34.
Spierings N., Holt DW., MacPhee IA. 2013. CYP3A5 genotype had no impact on intrapatient variability of tacrolimus clearance in renal transplant recipients. Ther Drug Monit. 35:328–31. DOI: 10.1097/FTD.0b013e318289644d. PMID: 23666583.

Htun YY., Swe HK., Saw TM. 2018. CYP3A5*3 genetic polymorphism and tacrolimus concentration in myanmar renal transplant patients. Transplant Proc. 50:1034–40. DOI: 10.1016/j.transproceed.2018.02.032. PMID: 29731062.

Rodriguez S., Gaunt TR., Day IN. 2009. Hardy-Weinberg equilibrium testing of biological ascertainment for mendelian randomization studies. Am J Epidemiol. 169:505–14. DOI: 10.1093/aje/kwn359. PMID: 19126586. PMCID: PMC2640163.

Hu R., Barratt DT., Coller JK., Sallustio BC., Somogyi AA. 2018. CYP3A5*3 and ABCB1 61A>G significantly influence dose-adjusted trough blood tacrolimus concentrations in the first three months post-kidney transplantation. Basic Clin Pharmacol Toxicol. 123:320–6. DOI: 10.1111/bcpt.13016. PMID: 29603629.
Zhang X., Liu ZH., Zheng JM., Chen ZH., Tang Z., Chen JS, et al. 2005. Influence of CYP3A5 and MDR1 polymorphisms on tacrolimus concentration in the early stage after renal transplantation. Clin Transplant. 19:638–43. DOI: 10.1111/j.1399-0012.2005.00370.x. PMID: 16146556.

Min SI., Kim SY., Ahn SH., Min SK., Kim SH., Kim YS, et al. 2010. CYP3A5 *1 allele: impacts on early acute rejection and graft function in tacrolimus-based renal transplant recipients. Transplantation. 90:1394–400. DOI: 10.1097/TP.0b013e3181fa93a4. PMID: 21076384.

Roy JN., Barama A., Poirier C., Vinet B., Roger M. 2006. Cyp3A4, Cyp3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharmacogenet Genomics. 16:659–65. DOI: 10.1097/01.fpc.0000220571.20961.dd. PMID: 16906020.

Chen JS., Li LS., Cheng DR., Ji SM., Sun QQ., Cheng Z, et al. 2009. Effect of CYP3A5 genotype on renal allograft recipients treated with tacrolimus. Transplant Proc. 41:1557–61. DOI: 10.1016/j.transproceed.2009.01.097. PMID: 19545678.

Rojas L., Neumann I., Herrero MJ., Bosó V., Reig J., Poveda JL, et al. 2015. Effect of CYP3A5*3 on kidney transplant recipients treated with tacrolimus: a systematic review and meta-analysis of observational studies. Pharmacogenomics J. 15:38–48. DOI: 10.1038/tpj.2014.38. PMID: 25201288.

Cheng Y., Li H., Meng Y., Liu H., Yang L., Xu T, et al. 2015. Effect of CYP3A5 polymorphism on the pharmacokinetics of tacrolimus and acute rejection in renal transplant recipients: experience at a single centre. Int J Clin Pract Suppl. (183):16–22. DOI: 10.1111/ijcp.12662. PMID: 26177012.
Li Y., Yan L., Shi Y., Bai Y., Tang J., Wang L. 2015. CYP3A5 and ABCB1 genotype influence tacrolimus and sirolimus pharmacokinetics in renal transplant recipients. Springerplus. 4:637. DOI: 10.1186/s40064-015-1425-5. PMID: 26543771. PMCID: PMC4628029.

Suzuki Y., Homma M., Doki K., Itagaki F., Kohda Y. 2008. Impact of CYP3A5 genetic polymorphism on pharmacokinetics of tacrolimus in healthy Japanese subjects. Br J Clin Pharmacol. 66:154–5. DOI: 10.1111/j.1365-2125.2008.03162.x. PMID: 18341670. PMCID: PMC2485247.

Fitzsimmons WE., Bekersky I., Dressler D., Raye K., Hodosh E., Mekki Q. 1998. Demographic considerations in tacrolimus pharmacokinetics. Transplant Proc. 30:1359–64. DOI: 10.1016/S0041-1345(98)00275-9. PMID: 9636552.

Passey C., Birnbaum AK., Brundage RC., Oetting WS., Israni AK., Jacobson PA. 2011. Dosing equation for tacrolimus using genetic variants and clinical factors. Br J Clin Pharmacol. 72:948–57. DOI: 10.1111/j.1365-2125.2011.04039.x. PMID: 21671989. PMCID: PMC3244642.

Bauer LA. Bauer LA, editor. 2008. Immunosuppressants. Applied clinical pharmacokinetics. 3rd ed. McGraw-Hill Medical;New York, NY: p. 682–711.
Fig. 1
Frequency (%) of CYP3A5*1 and CYP3A5*3 allele in renal transplant recipients (Myanmar ethnics, n=54).
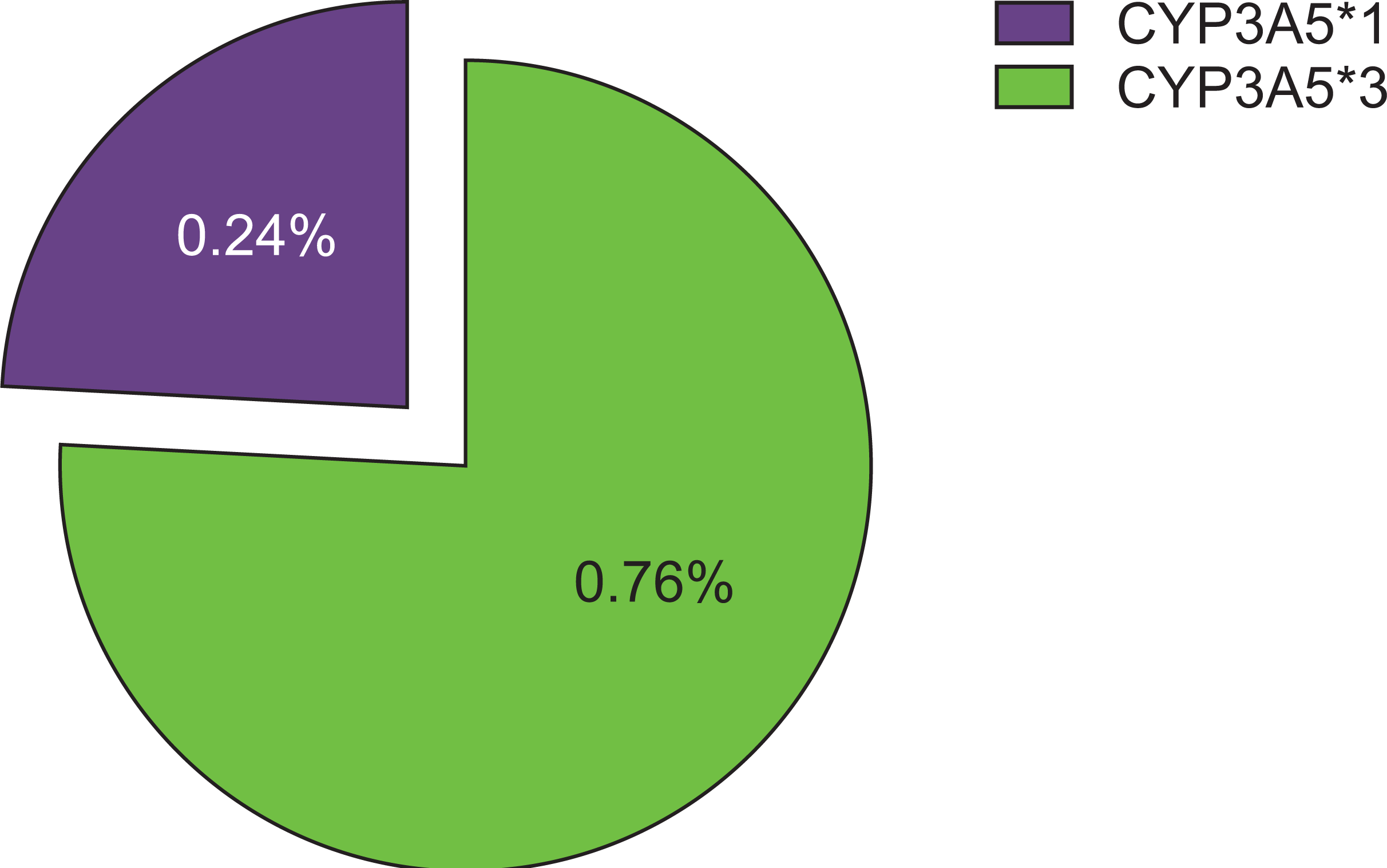
Fig. 2
(A) Comparison of mean trough concentration of tacrolimus between CYP3A5 non-expressor and expressor. (B) Comparison of mean tacrolimus concentration dose ratio (C/D) between CYP3A5 non-expressor and expressor. (C) Comparison of mean apparent clearance (CL/F) of tacrolimus between CYP3A5 non-expressor and expressor.
Table 1
Characteristics of CYP3A5 non-expressor and expressor
| Variable | CYP3A5 non-expressora) | CYP3A5 expressorb) |
|---|---|---|
| Mean age (yr) | 39.9±12.1 | 38.2±10.9 |
| Sex | ||
| Male | 20 | 16 |
| Female | 13 | 5 |
| Weight (kg) | 57.79±11.2 | 57.90±10.0 |
| Transplant | ||
| First | 33 | 21 |
| Second | 0 | 0 |
| Type of donor | ||
| Living related | 29 | 18 |
| Living unrelated | 4 | 3 |
| Cause of chronic kidney disease | ||
| Glomerulonephritis | 2 | 2 |
| Chronic pyelonephritis | 4 | 2 |
| Diabetic nephropathy | 4 | 3 |
| Hypertensive nephropathy | 18 | 11 |
| Undetermined | 3 | 2 |
| Others | 2 | 1 |
| Immunosuppressant use | ||
| Prednisone | 33 | 21 |
| Mycophenolate mofetil | 33 | 21 |
| Tacrolimus | 33 | 21 |
| Trough concentration (ng/mL) | 7.14±2.56 | 5.92±1.76 |
| Concentration dose ratio (ng/mL/mg) | 6.92±4.11 | 2.89±1.85 |
| Apparent clearance (L/hr) | 12.53±5.28 | 21.22±5.95 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download