Hwang S., Lee SG., Lee YJ., Sung KB., Park KM., Kim KH, et al. 2006. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl. 12:920–7. DOI:
10.1002/lt.20734. PMID:
16721780.

Hwang S., Lee SG., Ahn CS., Park KM., Kim KH., Moon DB, et al. 2005. Cryopreserved iliac artery is indispensable interposition graft material for middle hepatic vein reconstruction of right liver grafts. Liver Transpl. 11:644–9. DOI:
10.1002/lt.20430. PMID:
15915499.

Sugawara Y., Makuuchi M., Akamatsu N., Kishi Y., Niiya T., Kaneko J, et al. 2004. Refinement of venous reconstruction using cryopreserved veins in right liver grafts. Liver Transpl. 10:541–7. DOI:
10.1002/lt.20129. PMID:
15048798.

Hwang S., Lee SG., Song GW., Lee HJ., Park JI., Ryu JH. 2007. Use of endarterectomized atherosclerotic artery allograft for hepatic vein reconstruction of living donor right lobe graft. Liver Transpl. 13:306–8. DOI:
10.1002/lt.21045. PMID:
17256786.

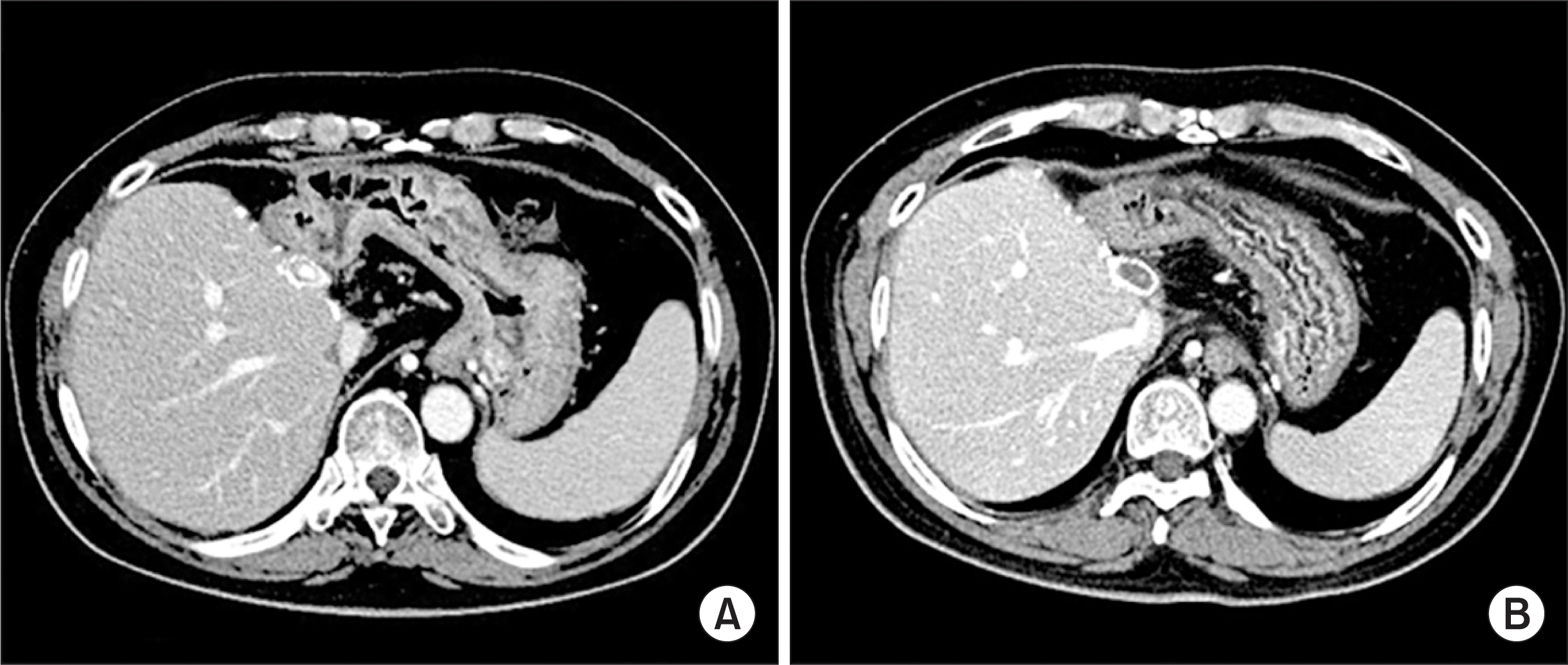
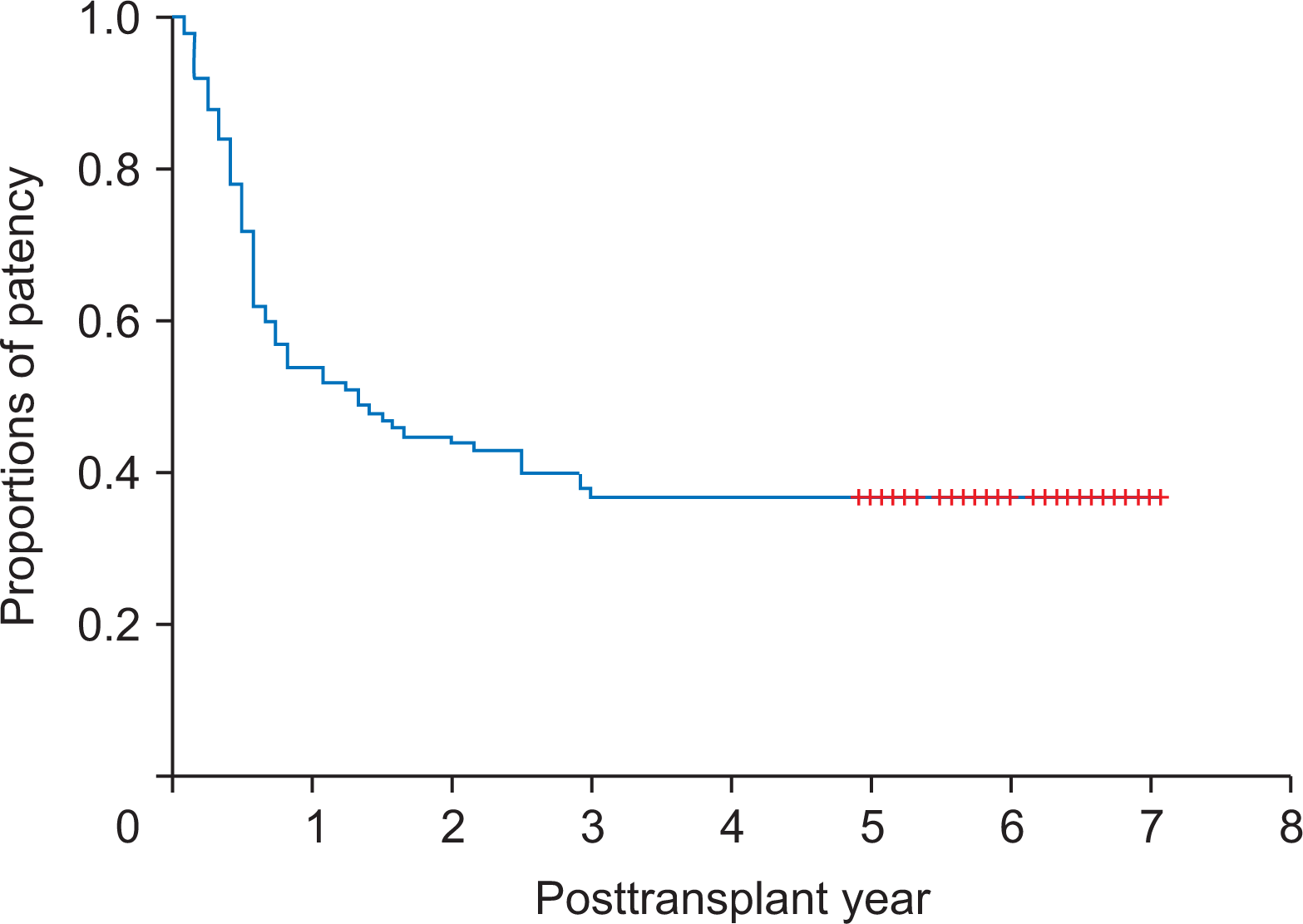
Hwang S., Jung DH., Ha TY., Ahn CS., Moon DB., Kim KH, et al. 2012. Usability of ringed polytetrafluoroethylene grafts for middle hepatic vein reconstruction during living donor liver transplantation. Liver Transpl. 18:955–65. DOI:
10.1002/lt.23456. PMID:
22511404.

Ha TY., Hwang S., Jung DH., Ahn CS., Kim KH., Moon DB, et al. 2014. Complications analysis of polytetrafluoroethylene grafts used for middle hepatic vein reconstruction in living-donor liver transplantation. Transplant Proc. 46:845–9. DOI:
10.1016/j.transproceed.2013.10.054. PMID:
24767363.

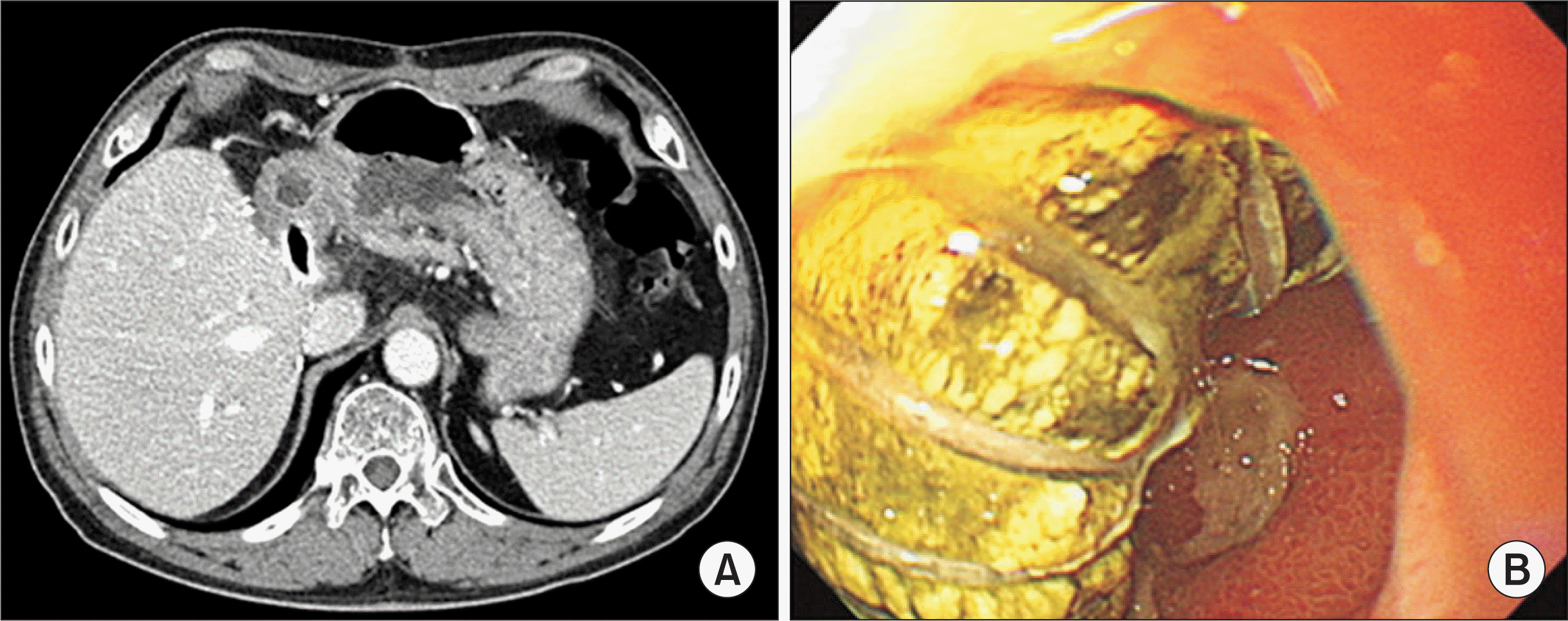
Hsu SC., Thorat A., Yang HR., Poon KS., Li PC., Yeh CC, et al. 2017. Assessing the safety of expanded polytetrafluoroethylene synthetic grafts in living donor liver transplantation: graft migration into hollow viscous organs. Diagnosis and treatment options. Med Sci Monit. 23:3284–92. DOI:
10.12659/MSM.902636. PMID:
28683053. PMCID:
PMC5510995.
Koc C., Akbulut S., Ozdemir F., Kose A., Isik B., Yologlu S, et al. 2019. Analysis of risk factors affecting the development of infection in artificial vascular grafts used for reconstruction of middle hepatic vein tributaries in living donor liver transplantation. Transplantation. 103:1871–6. DOI:
10.1097/TP.0000000000002583. PMID:
30747841.

Chung MH., Chuang CC., Liaw LF., Chen CY., Chen IM., Hsu CP, et al. 2018. Thrombotic ringed polytetrafluoroethylene graft with infection after living-donor liver transplantation. Transplant Proc. 50:2606–10. DOI:
10.1016/j.transproceed.2018.04.050. PMID:
30401360.

Goja S., Yadav SK., Roy R., Soin AS. 2018. A retrospective comparative study of venous vs nonringed expanded polytetrafluoroethylene etension grafts for anterior sector outflow reconstruction in right lobe living donor liver transplantation. Clin Transplant. 32:e13344. DOI:
10.1111/ctr.13344. PMID:
29981524.
Thorat A., Hsu SC., Yang HR., Li PC., Li ML., Yeh CC, et al. 2016. Reconstruction of isolated inferior right hepatic vein(s) in right lobe living donor liver transplantation using polytetrafluoroethylene grafts: a new feasible concept, technique of ¡®bridging conduit venoplasty¡¯ and outcomes. Ann Transplant. 21:735–44. DOI:
10.12659/AOT.900871. PMID:
27909288.

Ko GY., Sung KB., Yoon HK., Kim JH., Song HY., Seo TS, et al. 2002. Endovascular treatment of hepatic venous outflow obstruction after living-donor liver transplantation. J Vasc Interv Radiol. 13:591–9. DOI:
10.1016/S1051-0443(07)61652-2. PMID:
12050299.

Ko GY., Sung KB., Yoon HK., Kim KR., Kim JH., Gwon DI, et al. 2008. Early posttransplant hepatic venous outflow obstruction: long-term efficacy of primary stent placement. Liver Transpl. 14:1505–11. DOI:
10.1002/lt.21560. PMID:
18825710.

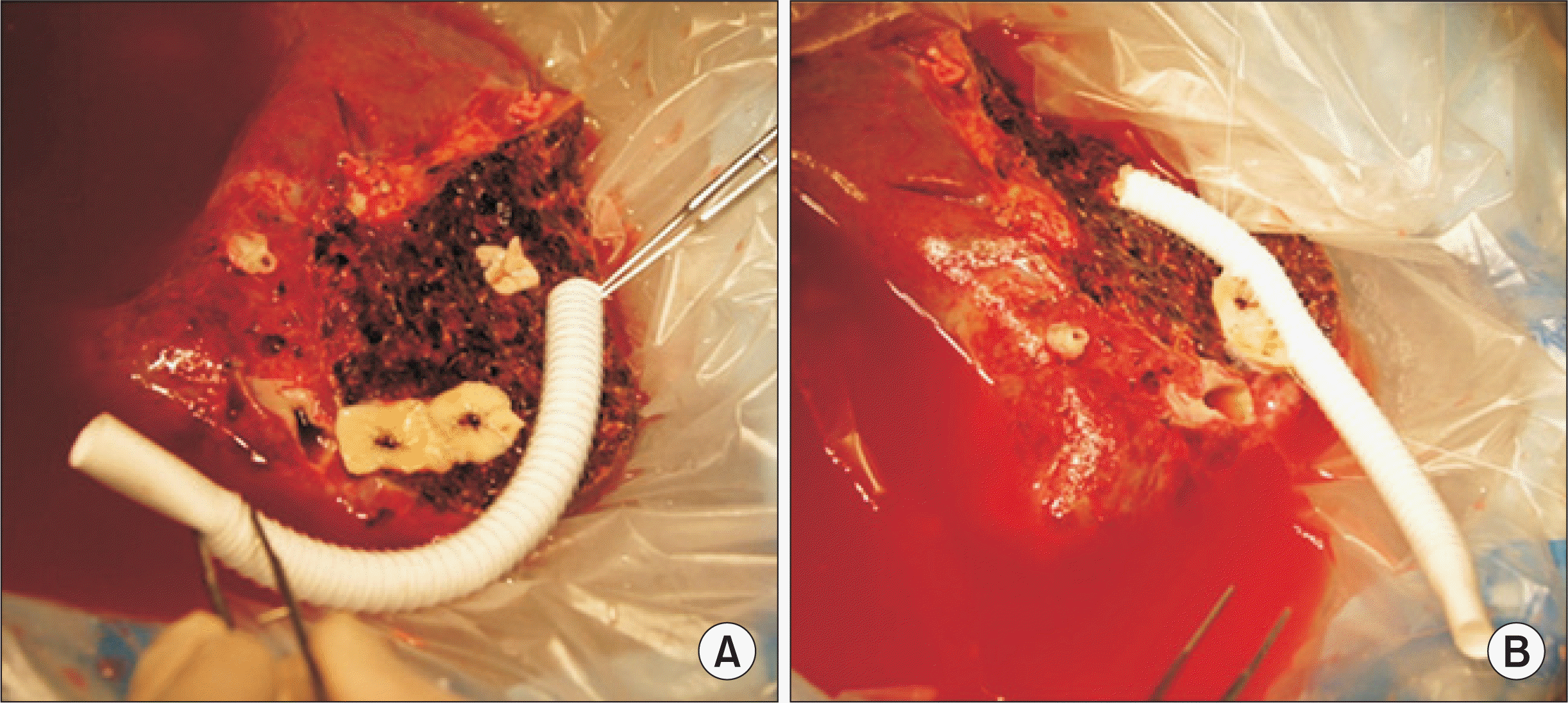
Jung DH., Lee SG., Hwang S., Hong HN. 2009. Feasibility assessment of interposition vessel graft substitutes in dog models for later clinical application to middle hepatic vein reconstruction during living donor liver transplantation. J Korean Surg Soc. 77:15–28. DOI:
10.4174/jkss.2009.77.1.15.

Papanicolaou G., Beach KW., Zierler RE., Detmer PR., Strandness DE Jr Jr. 1995. Hemodynamics of stenotic infrainguinal vein grafts: theoretic considerations. Ann Vasc Surg. 9:163–71. DOI:
10.1007/BF02139659. PMID:
7786702.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download