Abstract
Iatrogenic pancreatic duct injury can occur during resection of the choledochal cyst (CC). We herein present a case of postoperative pancreatic fistula (POPF) developed after resection of the CC in an adult patient with variant anomalous union of pancreatobiliary duct. The 55-year-old female patient underwent surgery after the diagnosis of CC-associated gallbladder cancer. During surgery, the CC mass was accidentally pulled out, by which the intrapancreatic CC portion was torn out from the main pancreatic duct. Since the pancreatic duct stump was not identified due to its small size, repair was not possible. The excavated defect at the pancreas head was closed securely combined with insertion of multiple drains. Postoperative POPF and peripancreatic fluid collection developed and the patient had to be fasted for 4 weeks. She was first discharged at 6 weeks after surgery. At 10 weeks, she was readmitted due to progression of peripancreatic fluid collection, which was controlled by percutaneous drain insertion. At 6 months, she was readmitted again due to repeated progression of peripancreatic fluid collection, which were controlled by endoscopic transmural duodenocystostomy. It took 8 months to resolve the pancreatic duct injury-associated pancreatitis. The experience in this case suggests that iatrogenic pancreatic duct injury during resection of CC can induce catastrophic complications, thus special attention should be paid to prevent pancreatic duct injury.
The choledochal cyst (CC) is often associated with anomalous union of the pancreatobiliary duct (AUPBD). Since the CC has malignant potential as well as development of de novo malignancy after incomplete resection, complete resection of CC is highly recommended in adult patients.1,2 However, the patterns of AUPBD are variable, thus there is a potential risk of iatrogenic pancreatic duct injury when surgeons attempt to remove the deeply-seated CC portion within the pancreas head. Such an iatrogenic pancreatic duct injury can lead to a major pancreatic leak, which is different from the usual postoperative pancreatic fistula (POPF) following pancreatoduodenectomy. We herein report our experience in the management of major POPF developed after resection of CC in an adult patient with variant AUPBD.
A 55-year-old female patient was admitted under the initial diagnosis of CC-associated gallbladder cancer. The computed tomography (CT) scan showed a highly suspected gallbladder cancer arising from the intracystic papillary neoplasm with the underlying CC type I (Fig. 1A). Magnetic resonance cholangiopancreatography (MRCP) showed intracystic papillary neoplasm of the gallbladder and CC combined with AUPBD type I (Fig. 1B). Fludeoxyglucose-positron emission tomography scan showed a definite hypermetabolic uptake at the gallbladder, suggesting malignant changes.
The extent of surgical resection was planned to be compatible with the extended cholecystectomy and CC resection with Roux-en-Y hepaticojejunostomy. After performing transection of the hepatic duct and extended cholecystectomy, dissection continued to remove the intrapancreatic cystic portion. At this time, the second assistant forcefully pulled out the CC mass, by which the intrapancreatic bile duct portion was accidentally torn out from the main pancreatic duct (Fig. 2). Since the pancreatic duct stump was not identified probably due to its small size, primary repair or internal stenting was not possible. After primary closure of the distal bile duct stump, the excavated defect at the pancreas head was closed securely with multiple sutures. Other parts of this CC surgery were similar to the standard procedures. Multiple Jackson-Pratt type abdominal drains and a 14-Fr pigtail catheter were inserted to cope with potential development of major POPF. The pathology report revealed that there was a 6 cm-sized moderately differentiated adenocarcinoma arising in the intracystic papillary neoplasm (Fig. 3). The depth of the gallbladder wall invasion was extension to the perimuscular connective tissue. There was no lymphovascular or perineural invasion. There was no malignant change at the CC specimen. One of the 3 regional lymph nodes were tumor-positive, making the tumor stage T2N1M0.
A water-clear pancreatic enzyme-rich fluid was drained up to 500 ml/day through the pigtail catheter, which was located at the pancreatic repair site. The dynamic CT scan taken after 5 days showed swelling of the pancreas with peripancreatic and right anterior pararenal space fluid collection, indicating development of acute pancreatitis (Fig. 4A). Acute pancreatitis was exacerbated and peripancreatic fluid collection increased on the 10-day CT scan (Fig. 4B). At the 4-week CT scan, pancreatitis and peripancreatic fluid collection began to resolve (Fig. 4C). Until this time point, the patient was permitted to have only sips of water under total parenteral nutrition. Pancreatitis was slowly improved with a marked decrease in pigtail drainage until 6 weeks (Fig. 4D). During these sequences, her general condition was good without febrile episodes. Thus, she was discharged after removal of the abdominal drains.
At the 10-week CT scan, there was noticeable progression of acute necrotic collection around the pancreas head, the small bowel mesentery, and along the right anterior pararenal space (Fig. 5A). She was readmitted and a new pigtail catheter was inserted percutaneously. At the 14-week CT scan, a liver abscess developed in the left liver (Fig. 5B). MRCP showed no abnormality at the intrahepatic bile duct and the remnant pancreatic duct was dilated with peripancreatic fluid collection (Fig. 5C). At the 17-week CT scan, the liver abscess improved and the walled-off necrosis around the pancreas gradually decreased (Fig. 5D). After removal of the pigtail catheter, she was discharged again.
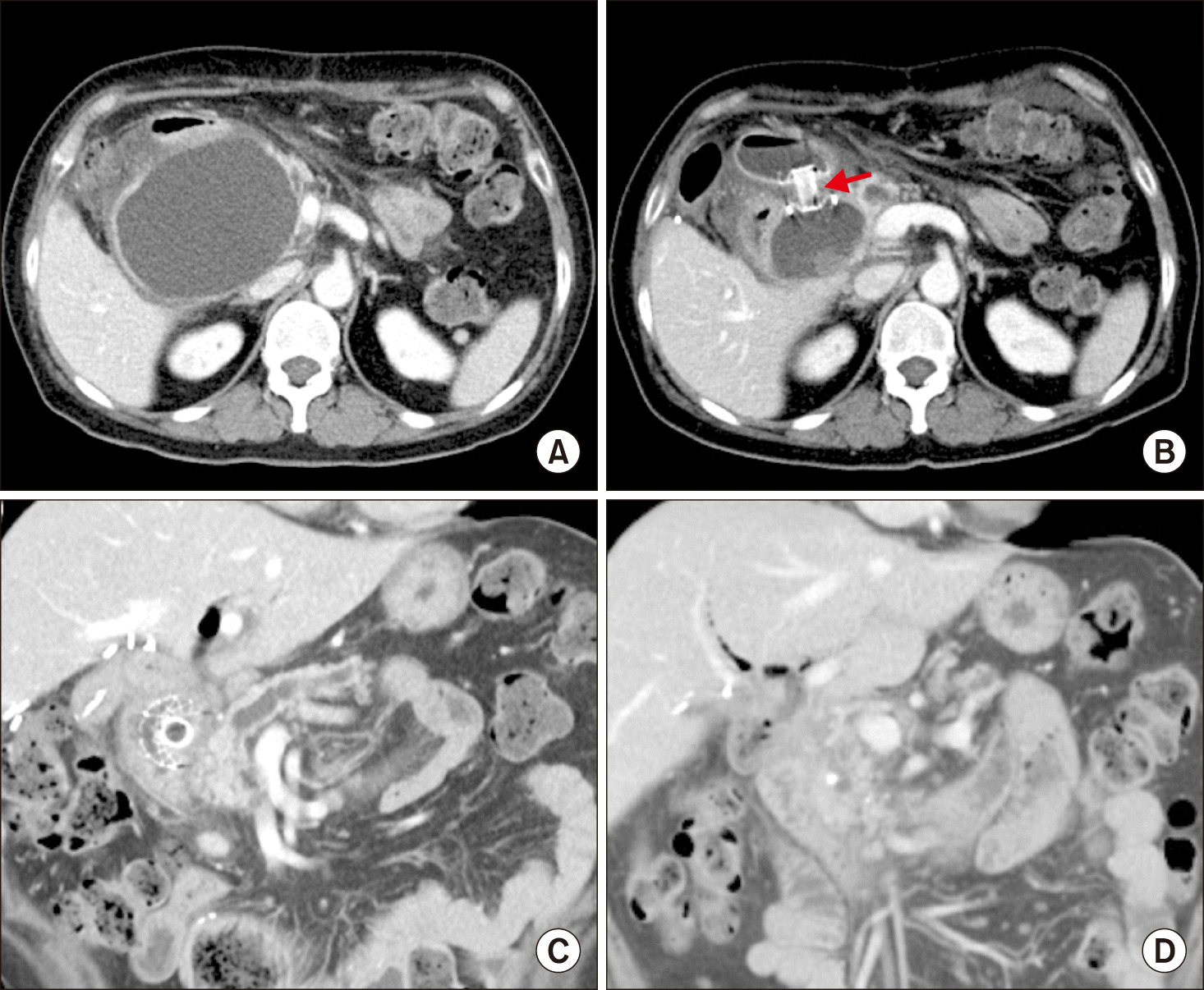
At the 6-month CT scan, there was a marked increase in complicated fluid collection posterior to the pancreatic head and the proximal duodenum (Fig. 6A). She was readmitted and endoscopic ultrasonography-guided transmural duodenocystostomy was performed to drain the peripancreatic fluid (Fig. 6B). She was discharged again for observation. After 2 months, she was readmitted to remove the wall stent. At the 8-month CT scan, there was a marked decrease in pancreatic pseudocyst, with further dilation of the pancreatic duct and atrophy of the pancreatic parenchyma (Fig. 6C). At the 12-month CT scan, there was no abnormal fluid collection and pancreatitis was completely controlled (Fig. 6D).
Adjuvant chemotherapy had been initially planned after surgery, but it was not performed due to delayed recovery from postoperative pancreatitis. Currently, she is doing well without any physical discomfort 13 months after the operation. She was diagnosed with new-onset type II diabetes during follow-up, thus administration of antidiabetic medication was necessary.
Complete resection of the CC has been the mainstay of the management of CC. However, some problems remain with intrahepatic and distal end parts of the CC. Although radical cyst excision is well known to be a treatment of choice, the risk of surgery-associated complications exist such as morbidity of the porta hepatis dissection and postoperative complications such as POPF and pancreatitis.3 As a result, surgeons occasionally tend to be reluctant to perform aggressive complete excision, especially in patients with unusual anatomical variations.
POPF is one of the most serious complication following surgical resection of the CC. Since CC is often buried deep within the pancreatic parenchyma, extensive dissection into the pancreatic parenchyma is an essential part of surgical procedure. It is important to protect the junction portion of the distal bile duct and pancreatic duct. If this portion is not definitely delineated at the preoperative CT or MRCP, special attention should be paid during pancreatic excavation. Meticulous CC dissection combined with intraluminal probing within the distal bile duct is a useful and intuitive surgical technique to prevent iatrogenic injury of the pancreatic duct.
In this case, we applied the abovementioned technique during dissection of the distal CC part. However, our second assistant accidentally pulled out the CC mass forcefully, by which the intrapancreatic cystic portion was torn out from the main pancreatic duct. We identified the injured junction portion of the distal bile duct and pancreatic duct because there was a small perforation at the torn-out site of the distal bile duct wall. However, we failed to identify the remnant pancreatic duct stump due to its small size. Since primary repair with internal stenting was not possible, the only method we could use was secure closure of the excavated pancreatic defect and insertion of multiple drains.
As anticipated, a major POPF and pancreatitis developed soon after surgery. We had to wait with supportive care and serial follow CT scan were repeated until the resolution of POPF. She fasted for 4 weeks under total parenteral nutrition and was discharged at 6 weeks after surgery. However, acute pancreatitis with marked fluid collection developed and gradually controlled with a pigtail drainage. At 6 months after the surgery, acute pancreatitis with peripancreatic fluid collection developed again. We supposed that initial acute pancreatitis from iatrogenic pancreatic duct injury was not completely controlled, thus pancreatitis was relapsed until marked atrophy of the pancreatic parenchyma. Since percutaneous drainage did not appear safe, endoscopic transmural duodenocystostomy was performed to remove the peripancreatic fluid collection, which was maintained for 2 months. It took at least 8 months to resolve the pancreatic duct injury-associated pancreatitis.
The present case was the only case where a patient suffered from pancreatic duct injury in the personal (SH) experience of one hundred cases of CC surgery. After reviewing this case retrospectively, we presumed that there was an unusual pattern of AUPBD, illustrated at Fig. 2. We had to transect the distal bile duct at the junction of the CC portion, but accidental torn-out of the pancreatic duct junction led to inevitable closure of the main pancreatic duct. On follow-up MRCP, we identified the existence of an accessory pancreatic duct inserted at the end of the distal bile duct. If such an accessory pancreatic duct was absent, we presume that pancreatitis might continue until complete atrophy of the pancreatic parenchyma.
Afraid of POPF, some surgeons were reluctant to perform aggressive complete resection of the intrapancreatic CC. We previously presented a case of cancer development at the remnant CC within the pancreatic parenchyma after 16 years, which was treated by pylorus-preserving pancreatoduodenectomy.4 In a Chinese study including 78 patients with partial resection of the CC, carcinogenesis in the residual intrapancreatic biliary duct developed in 14.1%.1 Development of cholangiocarcinoma more than 10 years after excision of CC is rare, with a median time of recurrence being 6 years.5-12 The risk of interval malignancy after excision of CC is known well, but there are no practical guidelines for long-term follow-up. Most patients diagnosed with cholangiocarcinoma long period after resection of the CC were not followed up routinely with radiological imaging and tumor markers.5
For diagnosis and treatment of CC, MRCP is the most important imaging study. Preoperative MRCP examination is essential to avoid damaging the pancreatic duct during surgery because it can visualize a specific morphology of the pancreatobiliary duct junction.13,14
The experience of our present case suggests that iatrogenic pancreatic duct injury during resection of CC can induce catastrophic complications, thus special attention should be paid to prevent pancreatic duct injury.
REFERENCES
1. Fan F, Xu DP, Xiong ZX, Li HJ, Xin HB, Zhao H, et al. 2018; Clinical significance of intrapancreatic choledochal cyst excision in surgical management of type I choledochal cyst. J Int Med Res. 46:1221–1229. DOI: 10.1177/0300060517728598. PMID: 29322850. PMCID: PMC5972235.

2. Cho MJ, Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, et al. 2011; Surgical experience of 204 cases of adult choledochal cyst disease over 14 years. World J Surg. 35:1094–1102. DOI: 10.1007/s00268-011-1009-7. PMID: 21360306.

3. Ando H, Kaneko K, Ito T, Watanabe Y, Seo T, Harada T, et al. 1996; Complete excision of the intrapancreatic portion of choledochal cysts. J Am Coll Surg. 183:317–321. PMID: 8843259.
4. Oh SY, Kwon JH, Hwang S. 2019; Development of adenocarcinoma at the remnant intrapancreatic cyst 16 years after resection of the choledochal cyst. Ann Hepatobiliary Pancreat Surg. 23:192–196. DOI: 10.14701/ahbps.2019.23.2.192. PMID: 31225424. PMCID: PMC6558127.

5. Ng DW, Chiow AK, Poh WT, Tan SS. 2016; Metachronous cholangiocarcinoma 13 years post resection of choledochal cyst-is long-term follow-up useful? a case study and review of the literature. Surg Case Rep. 2:60. DOI: 10.1186/s40792-016-0187-9. PMID: 27307284. PMCID: PMC4909682.

6. Shimamura K, Kurosaki I, Sato D, Takano K, Yokoyama N, Sato Y, et al. 2009; Intrahepatic cholangiocarcinoma arising 34 years after excision of a type IV-A congenital choledochal cyst: report of a case. Surg Today. 39:247–251. DOI: 10.1007/s00595-008-3825-4. PMID: 19280286.

7. Kumamoto T, Tanaka K, Takeda K, Nojiri K, Mori R, Taniguchi K, et al. 2014; Intrahepatic cholangiocarcinoma arising 28 years after excision of a type IV-A congenital choledochal cyst: report of a case. Surg Today. 44:354–358. DOI: 10.1007/s00595-012-0387-2. PMID: 23090140. PMCID: PMC3898144.
8. Nishiyama R, Shinoda M, Tanabe M, Masugi Y, Ueno A, Hibi T, et al. 2011; Intrahepatic cholangiocarcinoma arising 33 years after excision of a choledochal cyst: report of a case. Int Surg. 96:320–325. DOI: 10.9738/CC82.1. PMID: 22808614.
9. Ohashi T, Wakai T, Kubota M, Matsuda Y, Arai Y, Ohyama T, et al. 2013; Risk of subsequent biliary malignancy in patients undergoing cyst excision for congenital choledochal cysts. J Gastroenterol Hepatol. 28:243–247. DOI: 10.1111/j.1440-1746.2012.07260.x. PMID: 22989043. PMCID: PMC3816325.

10. Koike M, Yasui K, Shimizu Y, Kodera Y, Hirai T, Morimoto T, et al. 2002; Carcinoma of the hepatic hilus developing 21 years after biliary diversion for choledochal cyst: a case report. Hepatogastroenterology. 49:1216–1220. PMID: 12239908.
11. Suzuki S, Amao K, Harada N, Tanaka S, Hayashi T, Suzuki M, et al. 2004; A case of intrahepatic cholangiocarcinoma arising 26 years after excision of congenital biliary dilatation. Jpn J Gastroenterol Surg. 37:416–421. DOI: 10.5833/jjgs.37.416.

12. Ono S, Sakai K, Kimura O, Iwai N. 2008; Development of bile duct cancer in a 26-year-old man after resection of infantile choledochal cyst. J Pediatr Surg. 43:E17–E19. DOI: 10.1016/j.jpedsurg.2008.01.073. PMID: 18558159.

13. Yoon JH. 2011; Magnetic resonance cholangiopancreatography diagnosis of choledochal cyst involving the cystic duct: report of three cases. Br J Radiol. 84:e18–e22. DOI: 10.1259/bjr/77844300. PMID: 21172959. PMCID: PMC3473806.

14. Shimizu T, Suzuki R, Yamashiro Y, Segawa O, Yamataka A, Kuwatsuru R. 2001; Magnetic resonance cholangiopancreatography in assessing the cause of acute pancreatitis in children. Pancreas. 22:196–199. DOI: 10.1097/00006676-200103000-00014. PMID: 11249076.

Fig. 1
Preoperative radiologic findings. The computed tomography scan showed highly suspected gallbladder cancer arising from the intracystic papillary neoplasm with underlying choledochal cyst (A). Magnetic resonance cholangiopancreatography showed choledochal cyst combined with anomalous union of pancreatobiliary duct (B).
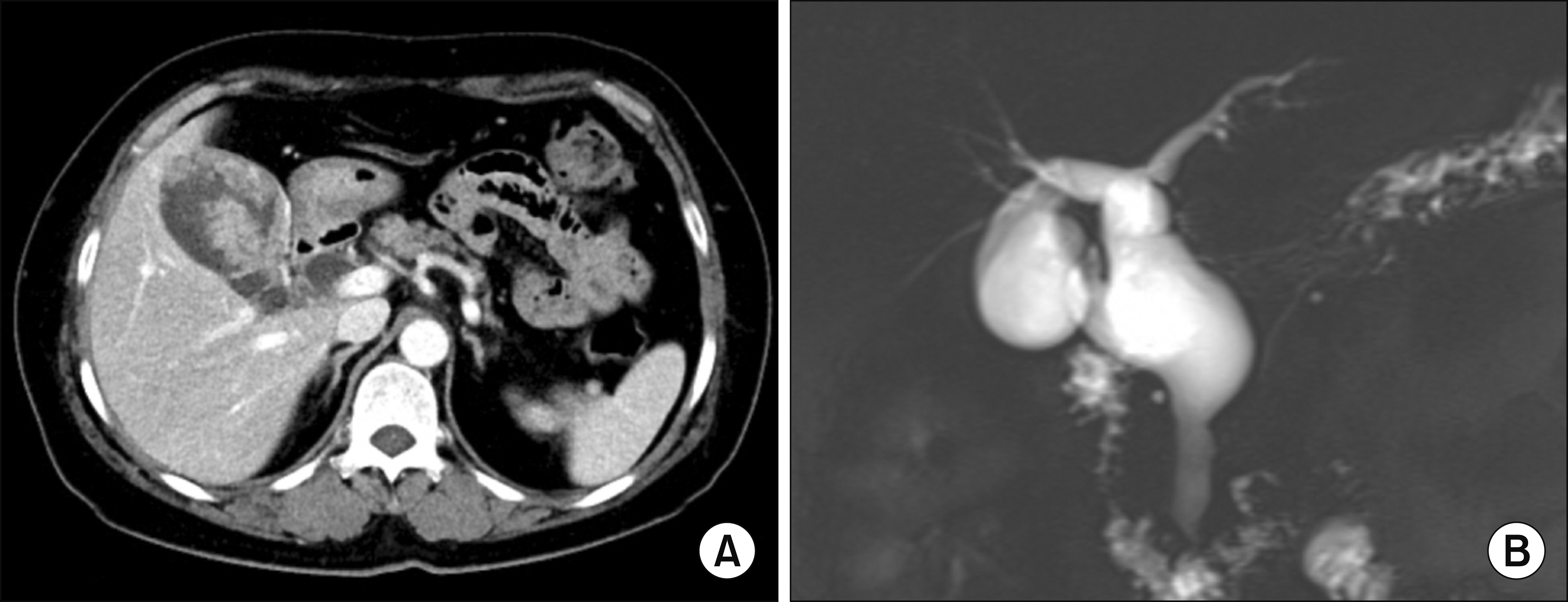
Fig. 2
The extent of resection overlapped at the magnetic resonance cholangiopancreatography image. A green bidirectional arrow was the preplanned transection line. The red thick dotted line indicates the main pancreatic duct and the blue thin dotted line indicates the accessory pancreatic duct. The yellow dotted area indicates the actual extent of resection.
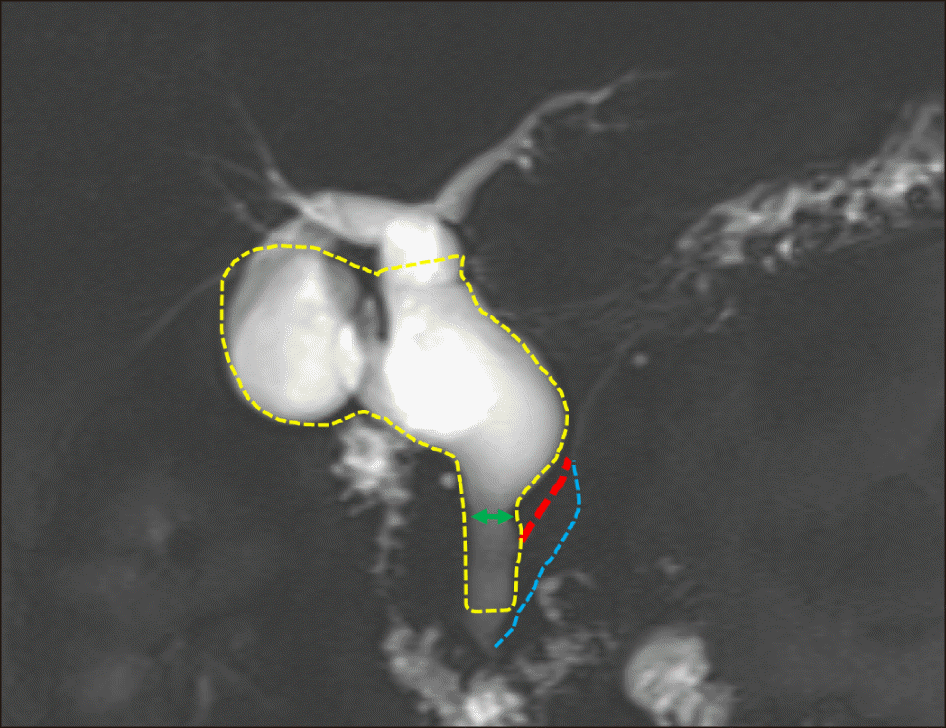
Fig. 3
Gross photographs of the resected specimen. There was a 6 cm-sized adenocarcinoma arising in the intracystic papillary neoplasm (A). The choledocal cyst was free of malignant changes (B).
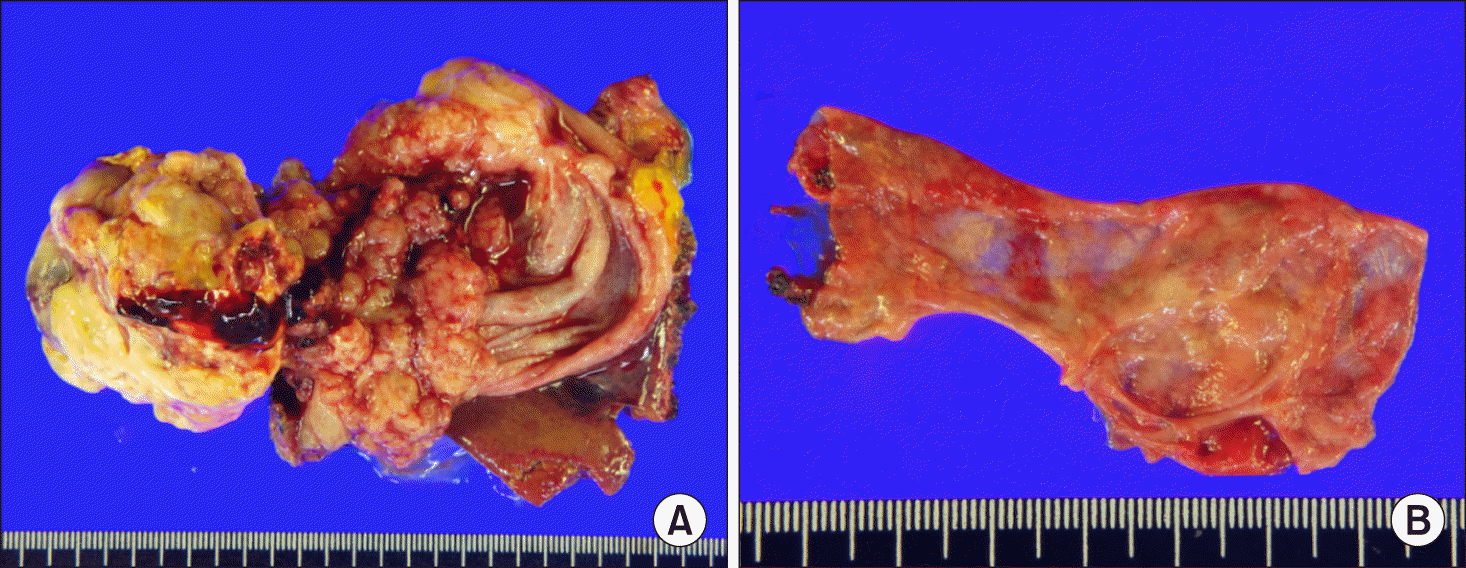
Fig. 4
Postoperative computed tomography (CT) findings. (A) The 5-day CT showed pancreatitis with peripancreatic fluid collection. (B) The 10-day CT showed exacerbation of acute pancreatitis. (C) The 4-week CT scan showed progressive resolution of pancreatitis and peripancreatic fluid collection. (D) The 6-week CT scan showed further improvement of pancreatitis.

Fig. 5
Postoperative computed tomography (CT) and magnetic resonance imaging findings. (A) The 10-week CT showed progression of peripancreatic necrotic collection. (B) The 14-week CT scan showed liver abscess at the left liver (arrow). (C) Magnetic resonance cholangiopancreatography image showed no abnormality at the intrahepatic bile duct and dilation of the remnant accessory pancreatic duct. (D) The 17-week CT showed resolution of liver abscess and improvement of peripancreatic necrosis.
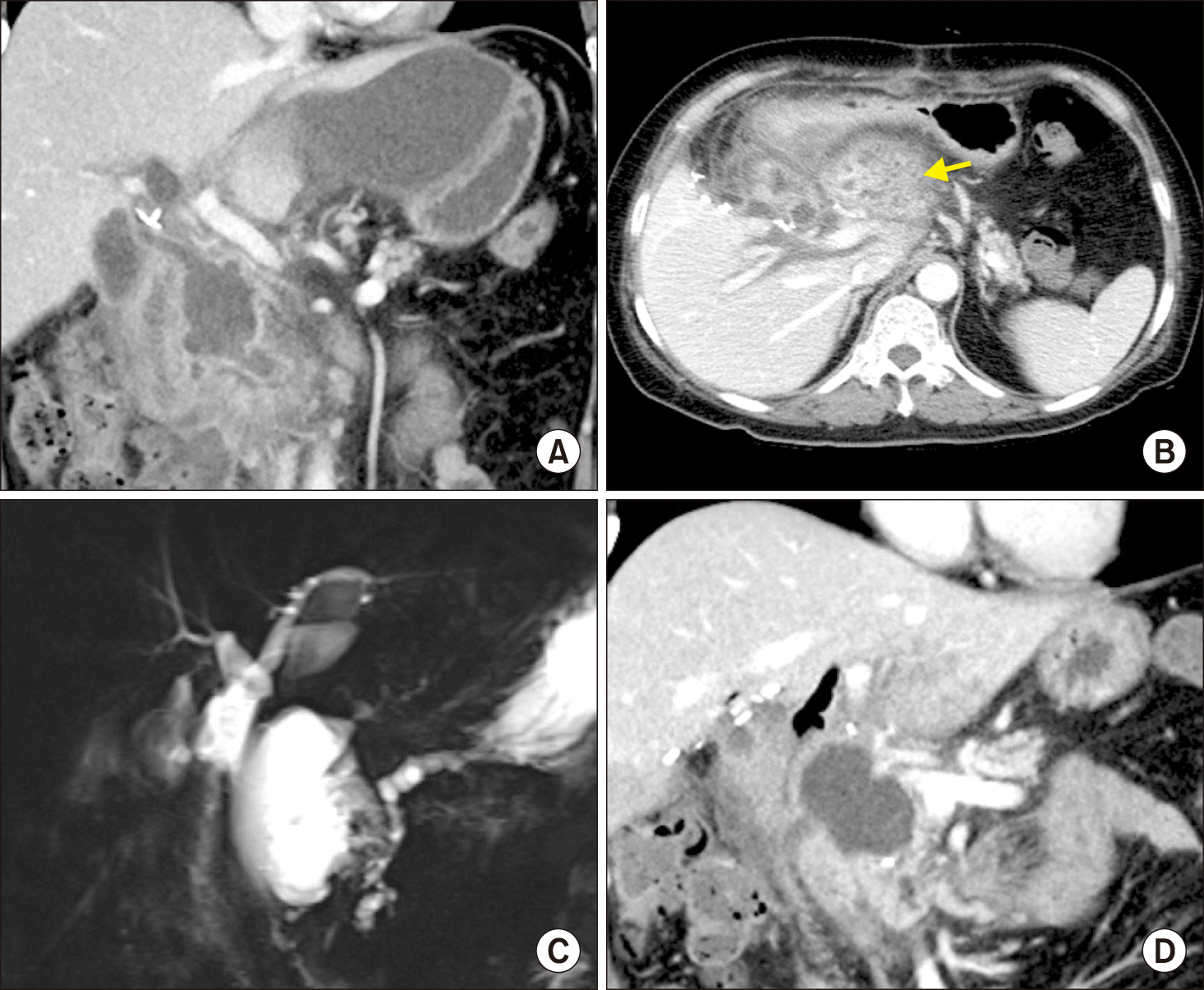
Fig. 6
Postoperative computed tomography (CT) findings. (A) The 6-month CT scan showed increased complicated fluid collection. (B) Endoscopic ultrasonography-guided transmural duodenocystostomy was performed to drain the peripancreatic fluid collection (arrow). (C) The 8-month CT scan showed marked decrease of pancreatic pseudocyst with further dilation of the pancreatic duct and atrophy of the pancreatic parenchyma. (D) The 12-month CT scan showed complete resolution of pancreatitis.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download