Abstract
Acute cellular rejection (ACR) after pediatric living donor liver transplantation (LDLT) is often curable with steroid pulse therapy, but a few pediatric patients show steroid-resistant ACR, which is difficult to control. We report the effect of everolimus as a rescue therapy for ACR in a case of pediatric LDLT. The patient was a 11-year-old girl who was admitted due to subacute liver failure of unknown cause. LDLT operation using a modified right liver graft from her mother was performed. The graft-recipient weight ratio was 1.30. The explant liver showed massive hepatic necrosis. The patient recovered uneventfully with immunosuppression using tacrolimus and low-dose steroid. However, at postoperative day (POD) 20, the liver enzyme levels began to increase. The first liver biopsy taken at POD 25 showed moderate ACR with rejection activity index (RAI) score of 7. At that time, steroid pulse therapy was performed, but the patient did not respond and the liver enzyme levels increased further. The second liver biopsy taken at POD 40 showed moderate ACR with RAI score of 7. At this time, everolimus was administered, and soon after that, liver enzyme levels had gradually improved. Currently, the patient is doing well for 44 months to date without any abnormal findings. The maintenance target trough concentrations were tacrolimus 5 ng/ml and everolimus 3 ng/ml. Our case demonstrated the effect of rescue therapy using everolimus for ACR following pediatric LDLT. Further studies are needed to assess the role of everolimus in pediatric liver transplant recipients suffering from ACR.
The prognosis of pediatric living donor liver transplantation (LDLT) is quite favorable, but a variety of graft rejections can develop at any time as like in the cases of adult liver transplantation (LT). It is noted that acute cellular rejection (ACR) after pediatric LDLT is often curable with administration of high-dose steroid pulse therapy, but a few pediatric patients show steroid-resistant ACR, which is known to be difficult to control and can be led to graft failure for this reason.
Everolimus, which is a mammalian target of rapamycin (mTOR) inhibitor, is a derivative of sirolimus with a similar mechanism of action. Sirolimus is a macrocyclic triene antibiotic that was initially found to have antifungal properties, but may also act as a primary immunosuppressant or antitumor agent.1 According the social health insurance policy in Korea, sirolimus is currently permissible to use for kidney transplantation and everolimus is permissible only for utilization with liver, heart and intestine transplantations. However, the safety and benefit of using everolimus in pediatric LT patients are not well known yet, although there are sporadic reports on pediatric cases using everolimus worldwide. Calcineurin inhibitor (CNI) inhibits calcium-dependent T-cell activation.2 In contrast, mTOR inhibitor acts on B cells independently of their effects on helper T cells, resulting in inhibition of antigen- and cytokine-driven B cell proliferation.3 There are many data demonstrating the valuable effects of mTOR inhibitors in adult solid organ transplant recipients.4-8 The synergism in efficacy between CNIs and mTOR inhibitors also allows significant reduction in CNI dosage.9,10 Because of such different modes of action, mTOR inhibitors have been used for control of refractory rejections in adult LT recipients.11,12 However, it is noted that there are only a few cases of pediatric LT cases using mTOR inhibitors for chronic rejection.13
Here we report the effect of everolimus as a rescue therapy for ACR in a case of pediatric LDLT.
The patient is a 11-year-old girl who was admitted due to having experienced jaundice for 2 months. Her initial blood laboratory findings were aspartate aminotransferase (AST) 508 IU/L, alanine aminotransferase (ALT) 434 IU/L, total bilirubin 20.5 mg/dl, direct bilirubin 15.5 mg/dl, and prothrombin time INR 2.11. After full work-up, she was finally diagnosed of acute liver failure of unknown cause (Fig. 1). The patient’s liver function did not recover despite being given the best supportive care for 20 days, thus LDLT operation was planned at that juncture.
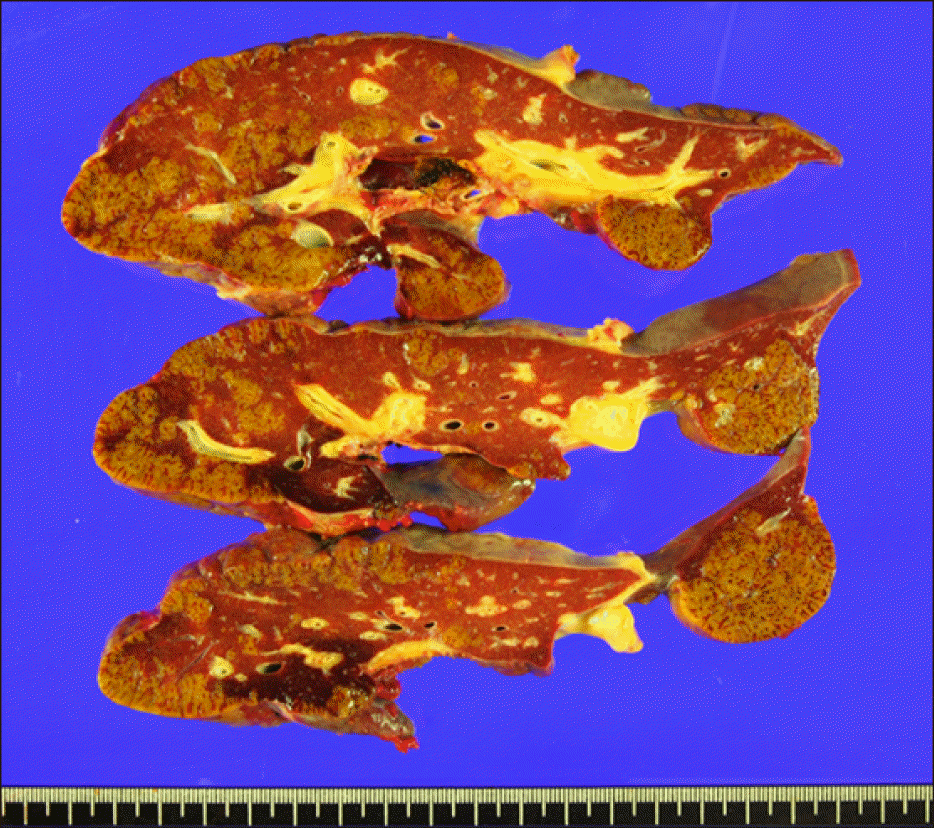
The donor was the patient’s mother. Since the patient weighed 44 kg, a modified right liver graft weighing 570 g was implanted according to the standard procedures of LDLT (Fig. 2A). The graft-recipient weight ratio was 1.30. Biliary reconstruction was performed through duct- to-duct anastomosis (Fig. 2B). The explant liver showed massive hepatic necrosis without portal inflammation (Fig. 3). The patient recovered uneventfully with immunosuppression using tacrolimus and low-dose steroid.
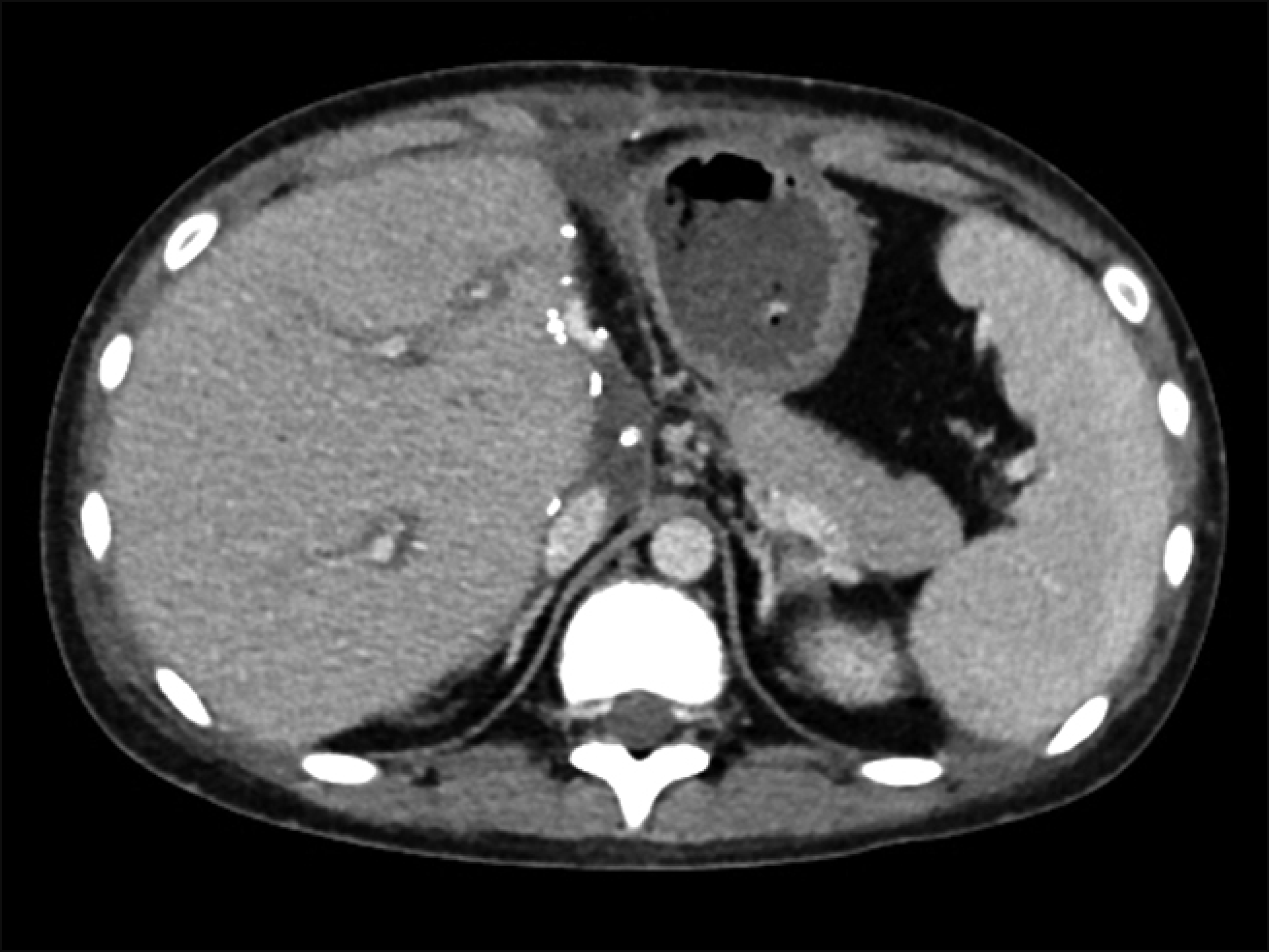
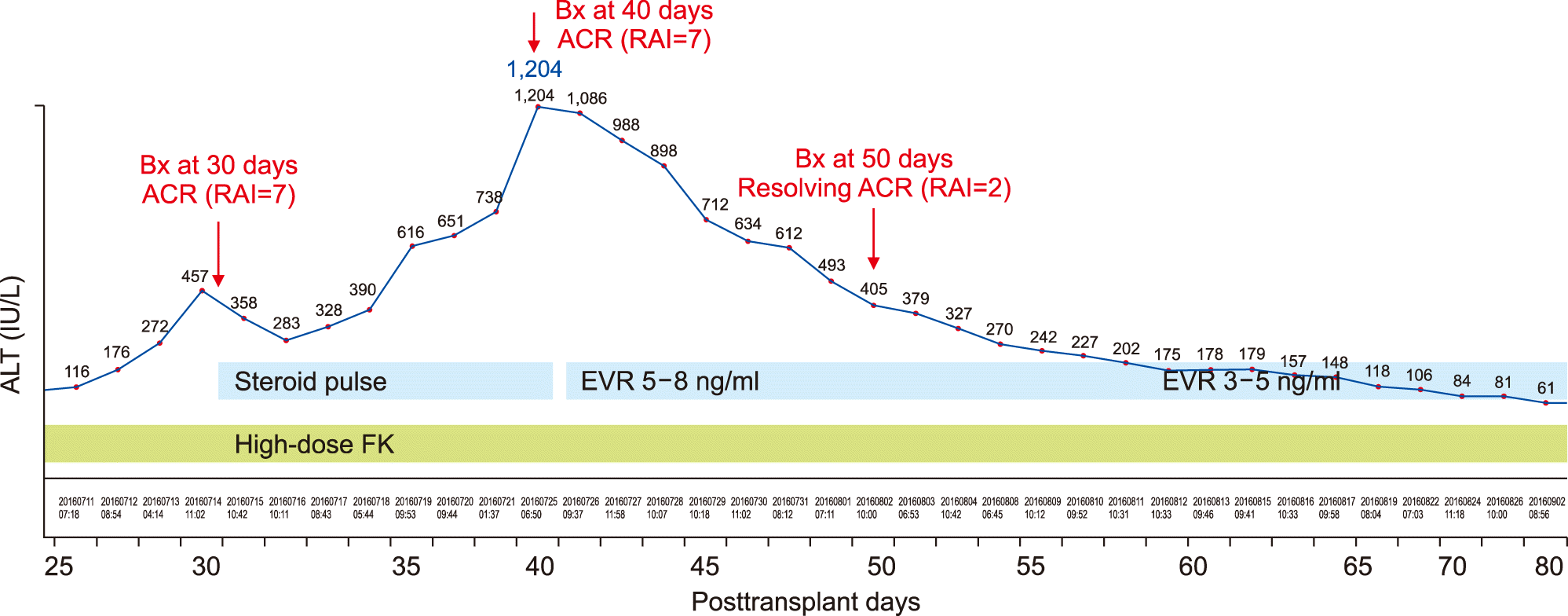
However, in this case, the liver enzymes began to increase after postoperative day (POD) 20. At POD 25, AST was raised to 457 IU/L, and thus a percutaneous liver biopsy was performed. The liver biopsy findings were as follows: moderate portal tract inflammation; endothelialitis with lymphocyte infiltration at perivenular hepatic parenchyma; multifocal hepatocyte apoptosis; and moderate lobular activity. These results were consistent with moderate ACR with a rejection activity index (RAI) score of 7. Steroid pulse therapy was performed, but the patient did not respond and liver enzyme levels increased further, reaching AST 1,024 IU/L at POD 40. Dynamic computed tomography scan showed swelling of the liver graft without vascular complications (Fig. 4). Additionally, the second liver biopsy showed severe portal tract inflammation, moderate bile duct damage, moderate endothelialitis, and perivenular hepatocyte dropout. These results were also consistent with moderate ACR with a RAI score of 7.
At this time, everolimus was administered as a rescue therapy to treat ACR. The initial target trough concentration of everolimus was 5-8 ng/ml. Soon after that, liver enzymes had gradually improved. At POD 50, the third liver biopsy was performed, in which there were mild portal tract inflammation, mild bile duct damage, mild canalicular and cholangiolar cholecystitis, and mild perivenular fibrosis and perisinusoidal and portal fibrosis. These were also consistent with resolving ACR with a RAI score of 2. Thereafter, the maintenance target trough concentration of everolimus was set to be 3-5 ng/ml. The serial changes of these clinical sequences were depicted at Fig. 5.
Currently, the patient is doing well for 44 months to date without any abnormal findings. The maintenance target trough concentrations were tacrolimus 5 ng/ml and everolimus 3 ng/ml.
The mTOR inhibitor is an immunosuppressant that interrupts the interleukin-2 proliferative response cascade and has dual inhibitory effects on cell growth and angiogenesis. mTOR inhibitor is increasingly administered as indicated for renal dysfunction or malignancy. The use of everolimus has been financially covered by the social health insurance in Korea since early 2016.14 Everolimus can be administer after first 1 month of LT primarily because of the potential risk of hepatic artery thrombosis and wound healing problems. However, everolimus is permissible to adult LT recipients in Korea and not yet to pediatric LT recipients. This is one of the main reasons why we presented this case.
Bilbao et al.11 reported that, as reviewed in 74 adult LT recipients, the causes of conversion to everolimus were reported to be refractory rejection in 31.1%, extended hepatocellular carcinoma in the explanted liver in 19%, hepatocellular carcinoma recurrence during follow-up in 8.1%, de novo tumor in 17.6%, CNI-related neurotoxicity in 10.8%, renal dysfunction in 8.1%, and others in 5.4%. They also reported that 73.9% of the refractory rejection cases were resolved after everolimus administration. When everolimus was used to treat refractory rejection (n=23), the event was resolved correctly in 17 patients (73.9%). The remaining six patients failed to respond: four progressed to severe chronic refractory rejection finally requiring retransplantation and two died, one due to sepsis and one from concomitant severe hepatitis C recurrence.
Ueno et al.13 also reported that chronic rejection in two pediatric LT recipients, in whom liver function tests were stable with no progression of liver biopsy fibrosis after everolimus administration. However, regarding the effect of everolimus on ACR in pediatric LT recipients, there was no report in the literature to our knowledge on this issue.
In Korea, everolimus is not permissible in pediatric LT recipients primarily due to the lack of information on safety of its administration, thus it has been rarely administered as a primary immunosuppressant to pediatric recipients. Nielsen et al.15 reported the first results using everolimus as a rescue therapy in pediatric LT recipients for the following indications: chronic graft dysfunction in 12, suspected CNI toxicity in 3, hepatoblastoma in 2, and recurrence of primary sclerosing cholangitis posttransplant in 1. They reported that four patients with chronic graft dysfunction recovered completely normal liver function tests using everolimus, six patients showed partial improvement, and two patients did not respond at all.
Worldwide, it is noted that administration of everolimus to pediatric LT recipients was reported from Germany by Nielsen et al.15 and Ganschow,16 France by Dumortier et al.,17 Japan by Ueno et al.,13 and in an international study from Germany, Sweden, United Kingdom and United States by Ganschow et al.18 With worldwide accumulation of experience of everolimus use in pediatric LT recipients, it is reasonable to permit everolimus administration for pediatric recipients by the Korea Ministry of Food and Drug Safety. However, many well-known side-effects and other published side effects of everolimus must be taken into account when administering everolimus to children and adolescents, namely they can experience the incidences of nausea and diarrhea, hematologic changes, and impaired fertility.9,19,20
In conclusion, our case demonstrated the effect of rescue therapy using everolimus for ACR following pediatric LDLT. Further studies are needed to assess the role of everolimus in pediatric LT recipients suffering from ACR, and the appropriateness of this treatment going forward for pediatric patient populations.
REFERENCES
1. Calne RY, Collier DS, Lim S, Pollard SG, Samaan A, White DJ, et al. 1989; Rapamycin for immunosuppression in organ allografting. Lancet. 2:227. DOI: 10.1016/S0140-6736(89)90417-0. PMID: 2568561.

2. Flanagan WM, Corthésy B, Bram RJ, Crabtree GR. 1991; Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 352:803–807. DOI: 10.1038/352803a0. PMID: 1715516.

3. Aagaard-Tillery KM, Jelinek DF. 1994; Inhibition of human B lymphocyte cell cycle progression and differentiation by rapamycin. Cell Immunol. 156:493–507. DOI: 10.1006/cimm.1994.1193. PMID: 7517796.

4. Holdaas H, Bentdal O, Pfeffer P, Mjørnstedt L, Solbu D, Midtvedt K. 2008; Early, abrupt conversion of de novo renal transplant patients from cyclosporine to everolimus: results of a pilot study. Clin Transplant. 22:366–371. DOI: 10.1111/j.1399-0012.2008.00795.x. PMID: 18279419.

5. De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, et al. H2304 Study Group. 2012; Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 12:3008–3020. DOI: 10.1111/j.1600-6143.2012.04212.x. PMID: 22882750. PMCID: PMC3533764.
6. Nashan B. 2018; mTOR inhibition and clinical transplantation: liver. Transplantation. 102(2S Suppl 1):S19–S26. DOI: 10.1097/TP.0000000000001690. PMID: 28230639.
7. Levy G, Schmidli H, Punch J, Tuttle-Newhall E, Mayer D, Neuhaus P, et al. 2006; Safety, tolerability, and efficacy of everolimus in de novo liver transplant recipients: 12- and 36-month results. Liver Transpl. 12:1640–1648. DOI: 10.1002/lt.20707. PMID: 16598777.

8. De Simone P, Carrai P, Precisi A, Petruccelli S, Baldoni L, Balzano E, et al. 2009; Conversion to everolimus monotherapy in maintenance liver transplantation: feasibility, safety, and impact on renal function. Transpl Int. 22:279–286. DOI: 10.1111/j.1432-2277.2008.00768.x. PMID: 19054383.

9. Saliba F, De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, et al. H2304 Study Group. 2013; Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 13:1734–1745. DOI: 10.1111/ajt.12280. PMID: 23714399.

10. Fischer L, Saliba F, Kaiser GM, De Carlis L, Metselaar HJ, De Simone P, et al. H2304 Study Group. 2015; Three-year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: follow-up results from a randomized, multicenter study. Transplantation. 99:1455–1462. DOI: 10.1097/TP.0000000000000555. PMID: 26151607.
11. Bilbao I, Dopazo C, Lazaro J, Castells L, Caralt M, Sapisochin G, et al. 2014; Multiple indications for everolimus after liver transplantation in current clinical practice. World J Transplant. 4:122–132. DOI: 10.5500/wjt.v4.i2.122. PMID: 25032101. PMCID: PMC4094947.

12. Mártinez JM, Pulido LB, Bellido CB, Usero DD, Aguilar LT, Moreno JL, et al. 2010; Rescue immunosuppression with mammalian target of rapamycin inhibitor drugs in liver transplantation. Transplant Proc. 42:641–643. DOI: 10.1016/j.transproceed.2010.02.011. PMID: 20304212.

13. Ueno T, Hiwatashi S, Saka R, Yamanaka H, Takama Y, Tazuke Y, et al. 2018; Everolimus rescue treatment for chronic rejection after pediatric living donor liver transplantation: 2 case reports. Transplant Proc. 50:2872–2876. DOI: 10.1016/j.transproceed.2018.03.079. PMID: 30318104.

14. Kang SH, Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, et al. 2019; Cross-sectional analysis of immunosuppressive regimens focused on everolimus after liver transplantation in a Korean high-volume transplantation center. Korean J Transplant. 33:98–105. DOI: 10.4285/jkstn.2019.33.4.98.

15. Nielsen D, Briem-Richter A, Sornsakrin M, Fischer L, Nashan B, Ganschow R. 2011; The use of everolimus in pediatric liver transplant recipients: first experience in a single center. Pediatr Transplant. 15:510–514. DOI: 10.1111/j.1399-3046.2011.01515.x. PMID: 21696525.

16. Ganschow R, Pape L, Sturm E, Bauer J, Melter M, Gerner P, et al. 2013; Growing experience with mTOR inhibitors in pediatric solid organ transplantation. Pediatr Transplant. 17:694–706. DOI: 10.1111/petr.12147. PMID: 24004351.

17. Dumortier J, Couchonnal E, Lacaille F, Rivet C, Debray D, Boillot O, et al. 2019; mTOR inhibitors in pediatric liver transplant recipients. Clin Res Hepatol Gastroenterol. 43:403–409. DOI: 10.1016/j.clinre.2018.11.010. PMID: 30528864.

18. Ganschow R, Ericzon BG, Dhawan A, Sharif K, Martzloff ED, Rauer B, et al. 2017; Everolimus and reduced calcineurin inhibitor therapy in pediatric liver transplant recipients: results from a multicenter, prospective study. Pediatr Transplant. 21. DOI: 10.1111/petr.13024. PMID: 28714558.

19. Lehmkuhl HB, Mai D, Dandel M, Knosalla C, Hiemann NE, Grauhan O, et al. 2007; Observational study with everolimus (Certican) in combination with low-dose cyclosporine in de novo heart transplant recipients. J Heart Lung Transplant. 26:700–704. DOI: 10.1016/j.healun.2007.02.008. PMID: 17613400.

20. Pascual J, Boletis IN, Campistol JM. 2006; Everolimus (Certican) in renal transplantation: a review of clinical trial data, current usage, and future directions. Transplant Rev. 20:1–18. DOI: 10.1016/j.trre.2005.10.005.

Fig. 1
Pretransplant computed tomography finding. The liver was shrunken and liver perfusion was impaired, suggesting a failing liver.
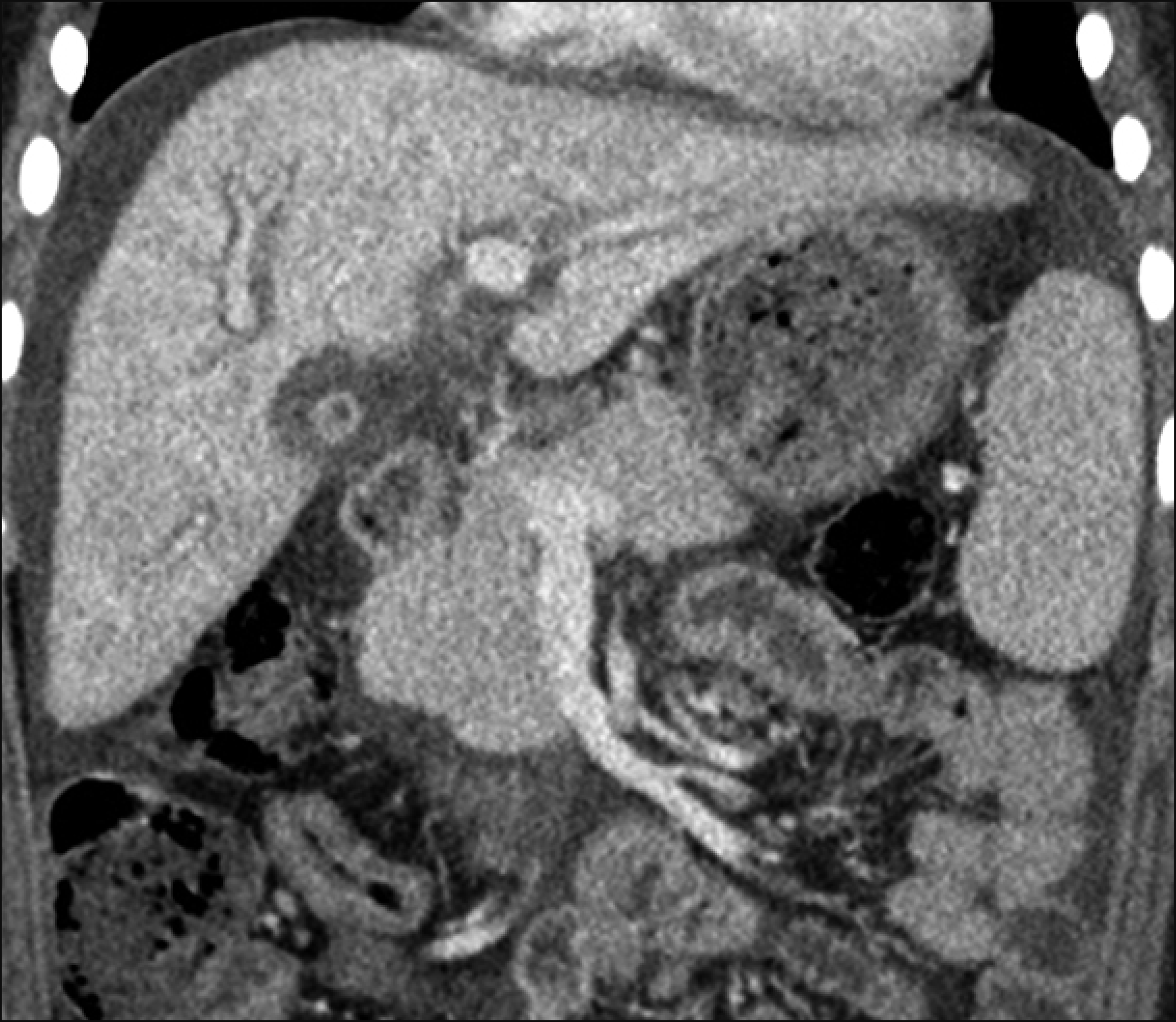
Fig. 2
Posttransplant findings. (A) Computed tomography scan taken 7 days after transplantation showed no abnormal findings. (B) Intraoperative cholangiogram showed uneventful duct-to-duct anastomosis.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download