Abstract
We report our first case of deceased-donor liver transplantation (LT) using a reuse liver graft after the first LT. The recipient was a 38-year-old female with fulminant hepatic failure from toxic hepatitis. She had a history of herb intake and her liver function deteriorated progressively. She was enrolled as the Korean Network for Organ Sharing (KONOS) status 1 and the model for end-stage liver disease score was 34. The donor was a 42-year-old male patient who fell into brain death after LT for alcoholic liver cirrhosis. Donation of multiple organs including the transplanted liver graft was performed 10 days after the first LT operation. Since the liver graft appeared to be normal and frozen-section liver biopsy showed only mild fatty changes, we decided to reuse the liver graft. A modified piggy-back technique of the suprahepatic inferior vena cava reconstruction was used. Other surgical procedures were comparable to the standard deceased-donor LT procedures. The explant liver pathology revealed submassive hepatic necrosis, which was compatible with toxic hepatitis. The peak of serum liver enzyme levels were aspartate transaminase 1,063 IU/L and alanine transaminase 512 IU/L at posttransplant day 3. Since the pretransplant general condition of the recipient was very poor, hospital stay was prolonged and she was discharged 51 days after LT operation. She is currently doing well for 3 years to date. Experience in our case and the literature review suggest that a reuse liver graft can be regarded as one of the marginal grafts which can be transplantable to the LT candidates requiring urgent LT.
The shortage of organ donors and the increased demand for liver transplantation (LT) have led to the widened concepts to increase the availability of liver grafts for LT. The acceptance of old and marginal liver donors, along with development of alternative techniques including liver graft splitting, living donors, and domino procedure, have been proposed to lower the mortality rate of patients on the waiting list.1 If a LT recipient experiences a fatal status of brain death, he or she can be a potential donor of single or multiple organs, including the transplanted liver.2-12 Such reuse liver grafts are regarded as marginal liver grafts, and they can be used as the life-saving grafts in LT candidates requiring urgent LT.
We report our first case of deceased donor LT using a reuse liver graft after the first LT operation.
The recipient was a 38-year-old female, blood group O, with fulminant hepatic failure from toxic hepatitis. She had a history of herb intake including arrowroot 1 month before and her liver function deteriorated progressively (Fig. 1). The laboratory findings at the time of waiting list registration was as following: serum creatinine 0.5 mg/dl, prothrombin time INR 4.6 and total bilirubin 15.6 mg/dl. Hepatitis B virus (HBV) surface antibody (anti-HBs) was positive with presence of HBV core antibody (anti- HBc) immunoglobulin G (IgG). She suffered from hepatic encephalopathy coma grade III-IV, thus ventilator support was applied at the time of waiting list registration. She was enrolled as the Korean Network for Organ Sharing (KONOS) status 1 because of fulminant hepatic failure. The model for end-stage liver disease score was 34. Three days later, a marginal liver graft was allocated for this patient.
The donor was a 42-year-old male patient with brain death. He had undergone LT using a whole liver graft from a brain-dead donor 10 days before because of alcoholic liver cirrhosis. This patient fell into brain death after LT operation. The donor had slightly elevated levels of serum liver enzymes and total bilirubin. Serum anti-HBs was negative and anti-HBc IgG was positive. Since the liver appeared to be normal and the frozen-section liver biopsy showed only mild fatty changes, we decided to reuse this liver graft. The liver, heart and one kidney were recovered from this donor.
After an inverted T-incision, routine surgical procedures for recipient hepatectomy were conducted. Since the liver graft was previously reconstructed by using the piggy-back technique in the first recipient, the recipient retrohepatic inferior vena cava (IVC) was completely preserved for application of the modified piggy-back technique.
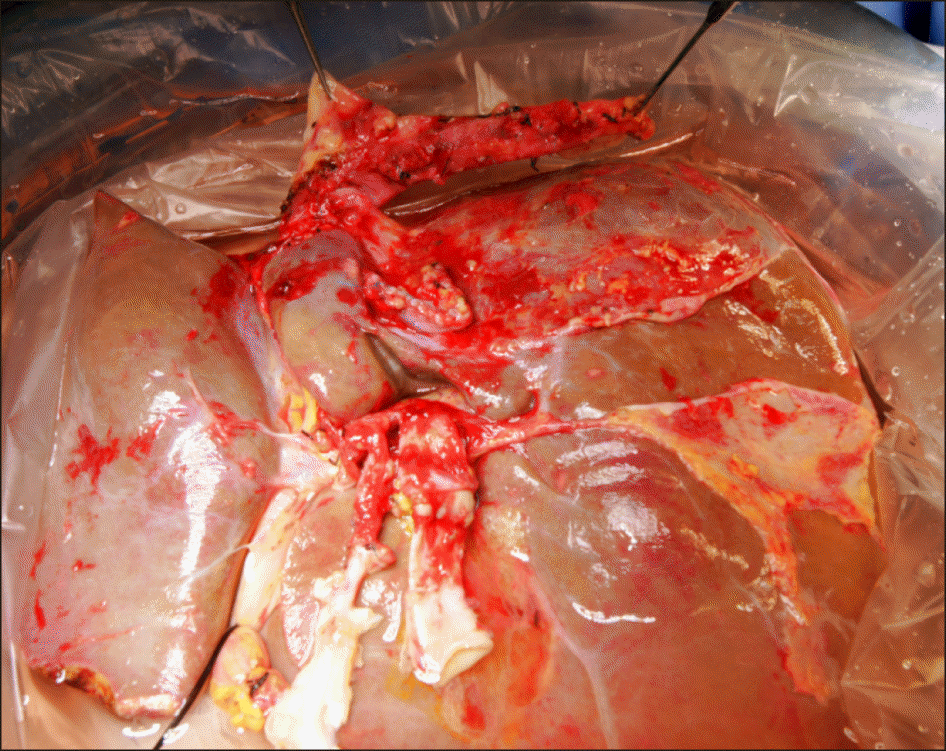
At the back table, the procured liver graft of 1,430 g in weight was processed for removal of the unnecessary structures (Fig. 2). The suprahepatic IVC was trimmed at the previous anastomosis line (Fig. 3A), thus no IVC portion from the first recipient was left. The infrahepatic IVC stump was already closed at the time of first LT (Fig. 3B). The main portal vein was transected at the previous anastomosis line (Fig. 3C, D). In contrast, the hepatic artery included a long arterial segment and an aortic patch of the first recipient (Fig. 3C, D).
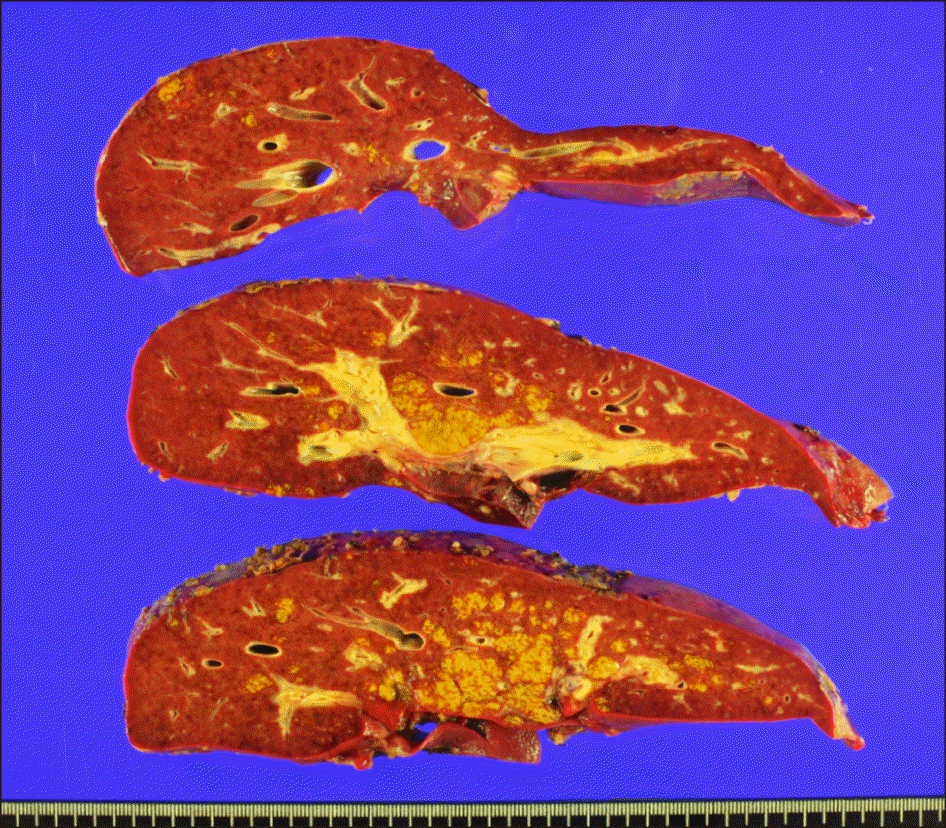
Since the patient suffered from fulminant hepatic failure, no collaterals were developed, active venovenous bypass using the dual inflow catheters from the common iliac vein and the main portal vein was performed. A modified piggy-back technique of suprahepatic IVC reconstruction was used (Fig. 4A). Additional 4 cm-long longitudinal incisions were made at both recipient and graft IVCs (Fig. 4B, C). These procedures made the IVC anastomosis sufficiently large (Fig. 4D). The main portal vein was reconstructed as end-to-end anastomosis (Fig. 5A). The redundant portion of the hepatic artery, which was derived from the first recipient, was resected and the graft’s own hepatic artery was anastomosed to the hepatic artery stump the second recipient (Fig. 5B). Biliary reconstruction was performed in duct-to-duct anastomosis of the common bile duct with T-tube insertion (Fig. 5B). The explant liver pathology revealed submassive hepatic necrosis, which was compatible with toxic hepatitis (Fig. 6).
At posttransplant day 1, the arterial resistive index of Doppler ultrasonography was lowered to 0.2, indicating arterial stenosis. Direct celiac arteriogram showed preserved hepatic arterial flow (Fig. 7A), thus the splenic artery was embolized to improve the hepatic arterial flow (Fig. 7B). The peak of serum liver enzyme levels were aspartate transaminase 1,063 IU/L and alanine transaminase 512 IU/L at posttransplant day 3. Since the pretransplant general condition of this patient was very poor, the hospital stay was prolonged and she was discharged 51 days after LT operation although no major posttransplant complication developed (Fig. 8) and recovery of the graft liver function was uneventful. She is currently alive for 3 years with co-medication of tacrolimus and mycophenolate mofetil. Since there was risk of reactivation of occult HBV infection from donor anti-HBc positivity, HBV immunoglobulin is administered every 4 months.
Organ shortage results in high rates of waiting list mortality and dropout of candidates for deceased donor LT. Although alternative techniques have been developed including the use of liver graft splitting, living donors, domino procedure, and marginal grafts, the number of available liver grafts does not meet the increasing demand.1 The number of deceased donors has been limited in Korea, many urgent LT candidates have died or undergone living donor LT.13,14
Reuse liver graft is regarded as one of the marginal donors. Depending on the urgency of LT candidates, most available marginal grafts can be used to save the LT candidates’ lives. This case was the 5465th case of overall LT and the first case of reuse LT in our institution. It was also the 824th case of adult deceased donor LT.
Ortiz et al.8 queried the United Network for Organ Sharing (UNOS) database for reuse graft LT, in which there were 11 cases from 1994 to 2003. The days from the first LT and graft procurement ranged from 1 day to 1,776 days; seven within 7 days and each one at 8 days, 17 days, 1,013 days and 1,776 days. Nine of the 11 grafts functioned well in the second recipient. Tayar et al.9 also reported a case of reuse LT after 13 years of the first LT.
Besides the recipients of deceased donor LT, the recipients of living donor LT can be reused. Hu et al.15 reported one case of successful reuse of extended right living donor liver graft after brain death of the first recipient. The first recipient, who had acute liver failure caused by hepatitis A virus infection, experienced brain death on the second day after LT. On the seventh day, the liver graft was procured with a patent hepatic artery, bile duct, portal vein, and reconstructed outflow and successfully implanted into the second recipient. The second recipient experienced a long-term survival of more than 8 years. 15
Experience in our case and the literature review suggest that the reuse liver graft can be include one of the marginal grafts which can be transplantable to the LT candidates requiring urgent LT.
REFERENCES
1. López-Navidad A, Caballero F. 2003; Extended criteria for organ acceptance. Strategies for achieving organ safety and for increasing organ pool. Clin Transplant. 17:308–324. DOI: 10.1034/j.1399-0012.2003.00119.x. PMID: 12868987.

2. Moreno EG, García GI, González-Pinto I, Gómez SR, Loinaz SC. 1991; Successful reuse of a liver graft. Br J Surg. 78:813–814. DOI: 10.1002/bjs.1800780715. PMID: 1873708.

3. Tantawi B, Cherqui D, Duvoux C, Dhumeaux D, Fagniez PL. 1996; Reuse of a liver graft five days after initial transplantation. Transplantation. 62:868–869. DOI: 10.1097/00007890-199609270-00029. PMID: 8824492.

4. Moreno González E, Gómez R, Gonzalez Pinto I, Loinaz C, Garcia I, Maffettone V, et al. 1996; Reuse of liver grafts after early death of the first recipient. World J Surg. 20:309–312. discussion 312–313. DOI: 10.1007/s002689900049. PMID: 8661836.
5. Figueras J, Pares D, González C, Ramos E, Rafecas A, Fabregat J, et al. 1997; Reuse of a transplanted liver. Transpl Int. 10:335–337. DOI: 10.1111/j.1432-2277.1997.tb00714.x. PMID: 9249947.

6. Pruvot FR, Roumilhac D, Dharancy S, Lambotte P, Auboiron A, Gambiez L, et al. 2004; Re-use of a liver graft and multi-organ procurement from a liver transplant patient. Transpl Int. 17:49–53. DOI: 10.1111/j.1432-2277.2004.tb00384.x. PMID: 14745488.

7. Rubay R, Wittebolle X, Ciccarelli O, Roggen F, Talpe S, Laterre PF, et al. 2003; Re-use of a liver allograft; an exceptional opportunity to enlarge the organ donor pool. Transpl Int. 16:497–499. DOI: 10.1111/j.1432-2277.2003.tb00355.x. PMID: 12712236.

8. Ortiz J, Reich DJ, Manzarbeitia C, Humar A. 2005; Successful re-use of liver allografts: three case reports and a review of the UNOS database. Am J Transplant. 5:189–192. DOI: 10.1111/j.1600-6143.2004.00635.x. PMID: 15636629.

9. Tayar C, Karoui M, Laurent A, Hadjhamida MB, Nhieu JT, Duvoux C, et al. 2006; Successful reuse of liver graft 13 years after initial transplantation. Transplantation. 82:1547–1548. DOI: 10.1097/01.tp.0000228238.40172.2f. PMID: 17164732.

10. Nafidi O, Letourneau R, Willems BE, Lapointe RW. 2007; Reuse of liver graft from a brain dead recipient. Clin Transplant. 21:773–776. DOI: 10.1111/j.1399-0012.2007.00724.x. PMID: 17988273.

11. Rentsch M, Meyer J, Andrassy J, Fischer-Fröhlich CL, Rust C, Mueller S, et al. 2010; Late reuse of liver allografts from brain-dead graft recipients: the Munich experience and a review of the literature. Liver Transpl. 16:701–704. DOI: 10.1002/lt.22053. PMID: 20517903.

12. Wong TC, She WH, Cheung TT, Chan SC, Lo CM. 2015; Case report of relay liver transplantation with graft infected with hepatitis B virus. Transplant Proc. 47:2768–2770. DOI: 10.1016/j.transproceed.2015.09.036. PMID: 26680090.

13. Cho WH. 2019; Organ donation in Korea in 2018 and an introduction of the Korea national organ donation system. Korean J Transplant. 33:83–97. DOI: 10.4285/jkstn.2019.33.4.83.

14. Ha HS, Hong JJ, Kim IO, Lee SR, Lee AY, Ha TY, et al. 2019; Deceased donor liver transplantation under the Korean model for end-stage liver disease score-based liver allocation system: 2-year allocation results at a high-volume transplantation center. Korean J Transplant. 33:112–117. DOI: 10.4285/jkstn.2019.33.4.112.

15. Hu XG, Kim IG, Wang HJ, Kim BW, Hong SY, Kim YB, et al. 2018; Reuse of living-donor liver graft in second recipient with long-term survival. Transplant Proc. 50:3984–3987. DOI: 10.1016/j.transproceed.2018.03.004. PMID: 30577301.

Fig. 1
Pretransplant imaging study findings. The liver was shrunken with development of ascites (A) with preservation of hepatic blood flow (B).

Fig. 3
Gross photograph of the bench work. (A) The suprahepatic inferior vena cava was trimmed at the previous anastomosis line. (B) The infrahepatic inferior vena cava stump was already closed at the time of first transplantation. (C) The main portal vein was transected at the previous anastomosis line. (D) The hepatic artery included a long arterial segment and aortic patch of the first recipient.
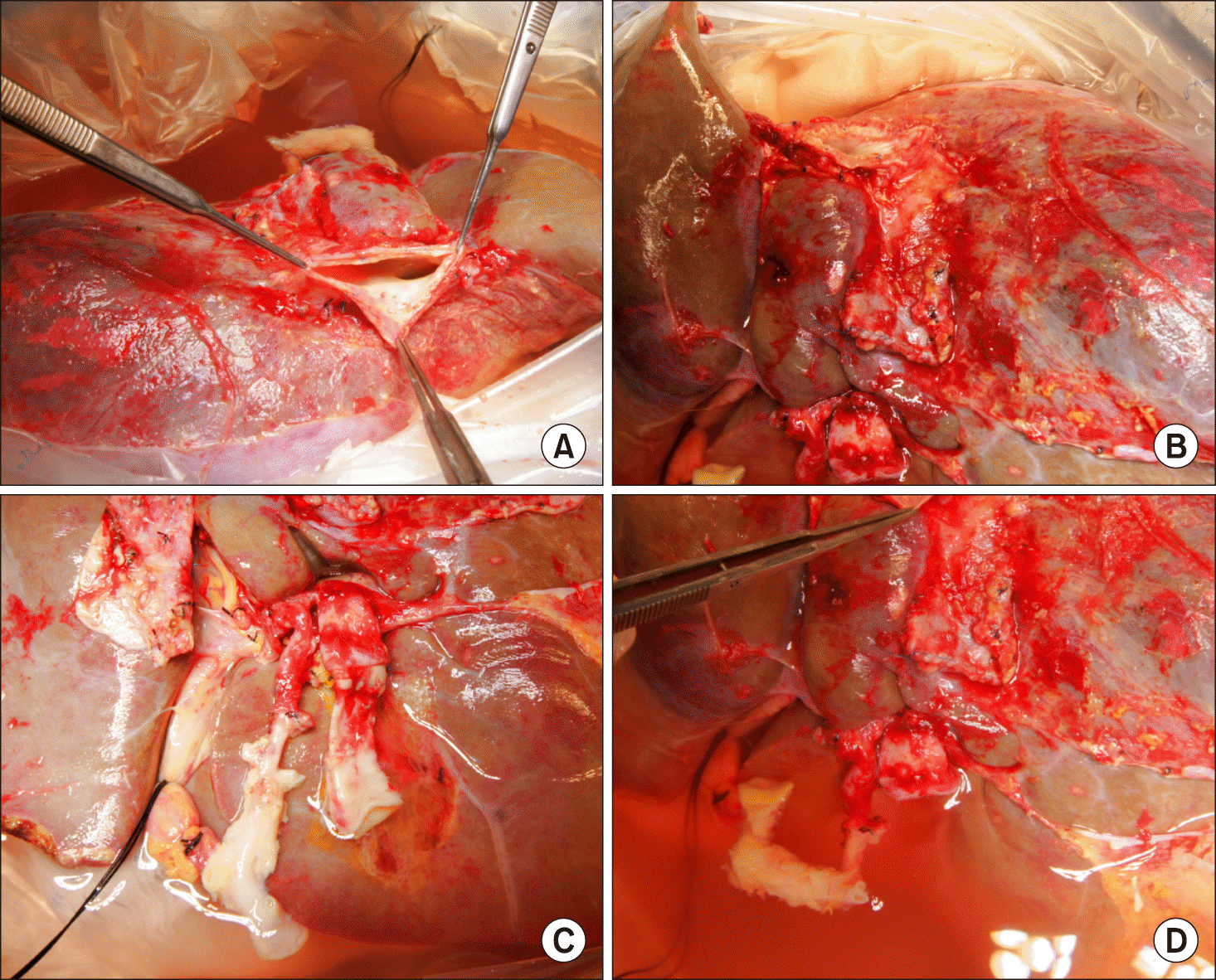
Fig. 4
Gross photograph of the inferior vena cava (IVC) reconstruction. (A) The IVC was totally clamped under active venovenous bypass. (B) A 4 cm-long longitudinal incisions were made at the caudal side of the recipient IVC opening. (C) A 4 cm-long longitudinal incisions were made at the caudal side of the graft IVC opening. (D) Two enlarged IVC openings were well matched, making a wide anastomosis opening.
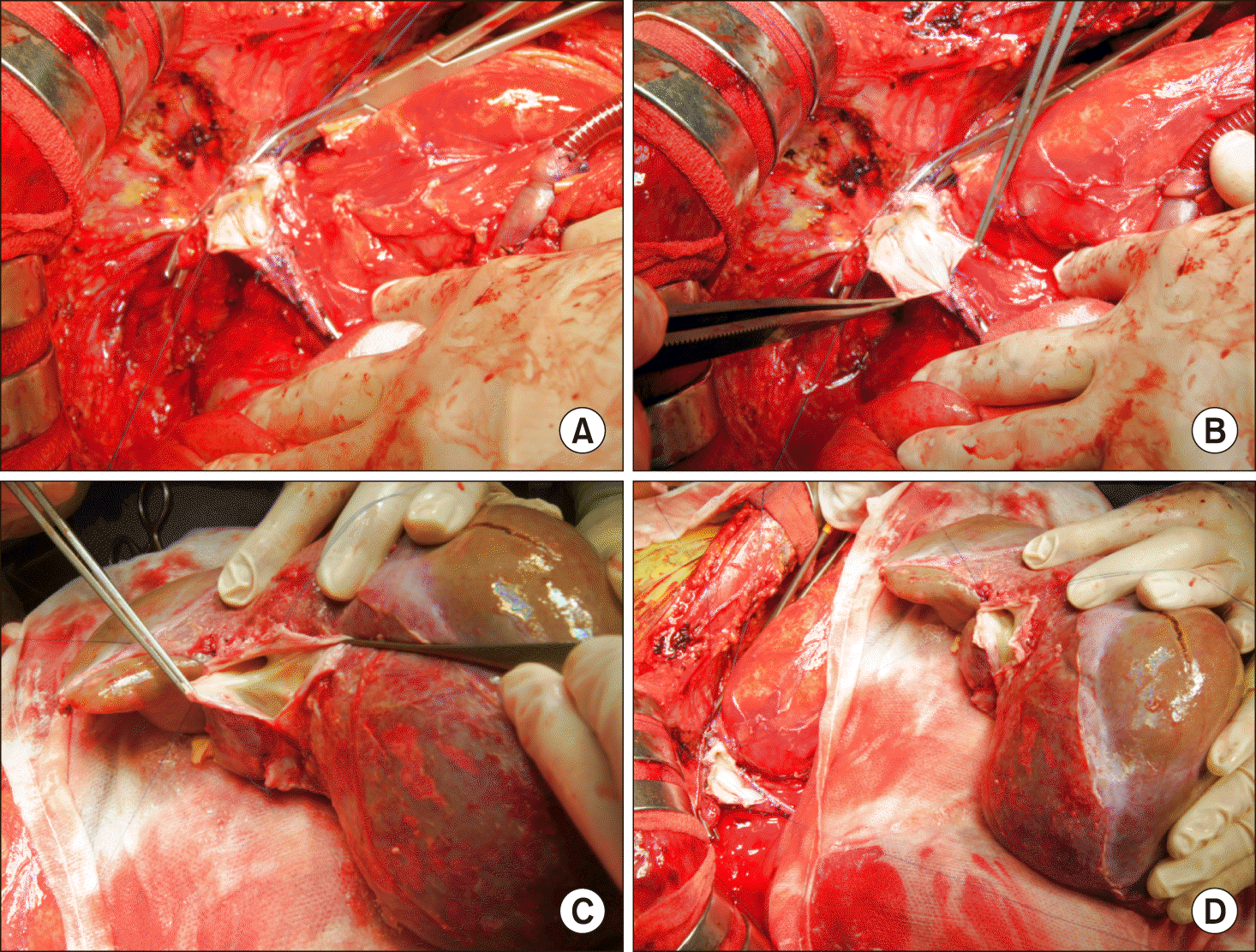
Fig. 5
Gross photograph of the hilar structure reconstruction. (A) The portal vein was reconstructed as end-to-end anastomosis. (B) The redundant portion of the hepatic artery was resected and the graft’s own hepatic artery was anastomosed to the recipient hepatic artery stump. Biliary reconstruction was performed in duct-to-duct anastomosis of the common bile duct with T-tube insertion.




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download