Abstract
Intrahepatic cholangiocarcinoma (ICC) accounts for 8-10% of all malignant liver tumors. Preponderance for elderly males and occurrence of varied morphological patterns in ICC is well known. Recent reports have described a newly recognized variant of thyroid-like cholangiocarcinoma. Herein, we present a hitherto unreported synchronous occurrence of an intrahepatic thyroid-like cholangiocarcinoma and a separate thyroid carcinoma in a 23-year-old post-partum woman. Both tumors displayed striking resemblance to follicular variant of papillary thyroid carcinoma (FVPTC) however exhibited disparate immunohistochemical profiles: the intrahepatic tumor was positive for CK7 and CK19, and negative for TTF-1, PAX-8 and thyroglobulin whereas, the thyroid tumor was positive for TTF-1, thyroglobulin and PAX-8. Young age, female proclivity, large mass at presentation and unique histology in thyroid-like ICC hint towards a distinctive subset of ICC. Awareness and recognition of this rare entity is essential, not only for accurate diagnosis, but also for gathering information on its biology and clinical behavior. Synchronous occurrence with a FVPTC is a challenging scenario that can simulate metastatic disease and mislead subsequent patient management. Whether morphologic similarity points to an underlying linkage between the two different tumors needs exploration.
‘Orphan Annie eye nuclei’ were once considered synonymous with papillary carcinoma of thyroid1,2 however, recently many new entities of “thyroid-like” carcinomas (mainly indicating follicular pattern and optically clear nuclei) are increasingly being reported in head and neck region (cribriform adenocarcinoma of tongue),3 kidney,4 and breast (thyroid-like carcinoma).5 These tumors have a striking semblance to follicular variant of papillary thyroid carcinoma, the possibility of metastasis from which needs exclusion in such cases. With the recent report of two separate cases, first one by Fornelli et al.6 and the second one by Chablé-Montero et al.,7 intrahepatic cholangiocarcinoma too has secured a place in the expanding list of sites for ‘thyroid-like’ carcinomas outside the thyroid. Also, in the article by Fornelli et al.,6 they have described a case reported by Foucar et al.8 in 1979, which was reported to be an unusual variant of cholangiocarcinoma in a 27 year old pregnant woman, with histological features mimicking those of ‘thyroid-like cholangiocarcinoma’ reported by Fornelli et al.6 Occurrence of a thyroid-like intrahepatic cholangiocarcinoma with a synchronous thyroid carcinoma has not been reported in English literature, to the best of our knowledge. Herein, we present, a hitherto unreported case of thyroid-like cholangiocarcinoma with coexistent thyroid carcinoma in a 23-year-old woman. Owing to the similar histology of both tumors, a possibility of metastasis from either site to the other was clinically more conceivable. However, distinctive, non-overlapping immunohistochemical profiles clearly identified both the tumors as two synchronous primaries. We discuss this case for not only its rarity but also for the dilemmas and challenges entailed in its diagnosis.
A 23-year-old woman was detected to have an abdominal lump while being evaluated for post-partum menorrhagia. A large firm mass was palpable in the epigastric region, 6 cm below the costal margin. On a triphasic computed tomography scan, a well-defined, capsulated heterogenous lesion, measuring 8.6×7.5 cm was identified in segments II and III of the left lobe of liver. The lesion was showing intense heterogenous contrast enhancement in arterial phase as compared to the surrounding liver parenchyma; contrast enhancement in portal and venous phases was similar to remaining liver parenchyma. On magnetic resonance imaging, the mass appeared well encapsulated and had solid enhancing areas (on post-contrast scans) as well as multiple non-enhancing cystic areas of varying sizes. Radiological differential diagnoses included atypical focal nodular hyperplasia and a sarcoma of the liver (primary or metastatic). Viral markers were non-reactive; serum alpha-feto protein and carcinoembryonic antigen levels were within normal limits. Liver function tests were normal except for a mild elevation in alkaline phosphatase levels. The patient underwent left hepatectomy.
On gross evaluation, a circumscribed mass measuring 12.1×9×6.3 cm was seen in the left lobe of liver. The liver capsule was intact. The cut surface was solid-cystic with areas of hemorrhage. On microscopy, the tumor was nodular and partly encapsulated by a thick fibrous capsule at places, while in other areas tumor cells directly infiltrated the surrounding liver parenchyma. The most eye-catching feature of the tumor was a diffuse follicular architecture with follicles filled with colloid-like eosinophilic material and lined by overlapping, ‘optically clear’ nuclei bearing grooves (Fig. 1, upper panel). The neoplastic follicles were disposed as micro- (20%) and macro-follicles (80%). The nuclear features and follicular architecture was strikingly similar to that of a follicular variant of papillary carcinoma thyroid (FVPTC). Intranuclear inclusions were however conspicuous by their absence. Negligible stroma was present between the tumor follicles. The tumor was reaching the liver capsule at places, without breaching it. Lymphovascular emboli were identified, within and outside the tumor boundary. Mitoses were infrequent (2/10 hpf) and necrosis was not seen. The background liver parenchyma was unremarkable and non-cirrhotic. Hepatic parenchymal margins and lymphnodes were uninvolved. Immunohistochemistry (IHC) was performed using automated immunostainer (Benchmark XT, Ventana, Roche) for antibodies to CK7, CK19, TTF-1, PAX-8, and HBME-1. Tumor cells were diffusely and strongly CK7 positive; CK19 was positive in approximately 60% of cells with luminal accentuation pattern of positivity. TTF-1, Thyroglobulin, PAX-8 and HBME-1 were negative in the tumor cells (Fig. 2, upper panel). Although the IHC profile was incompatible with a thyroid origin, due to a striking morphological resemblance to the same, an ultrasound of the thyroid was performed.
Thyroid sonography showed a hypoechoic nodule with ill-defined borders of size 9×6 mm in the right lobe of thyroid showing internal vascularity and microcalcifications. Fine needle aspiration cytology was reported as suspicious for papillary carcinoma (Bethesda category V) for which the patient underwent a total thyroidectomy with central compartment clearance.
Thyroid specimen, weighing 18 grams, revealed a 0.8 cm in diameter, grey, firm to hard nodule. Histology revealed a micro-carcinoma (8 mm in greatest dimension) with features of FVPTC (Fig. 1, lower panel). The tumor was unencapsulated, unifocal, and without lymphovascular emboli or extrathyroidal extension. Surgical margins and lymph nodes were uninvolved. On IHC, thyroid tumor was positive for all thyroid lineage markers including TTF-1, thyroglobulin, PAX-8, CK19 and HBME1, which was clearly disparate from the hepatic tumor (Fig. 2, lower panel).
Based on distinct and contrasting IHC profiles of the two tumors with identical morphology of FVPTC, a diagnosis of synchronous thyroid-like intrahepatic cholangiocarcinoma and follicular variant of papillary thyroid (micro) carcinoma was made in this case. Patient is alive and disease free 18 months after hepatic surgery.
Recently, carcinoma with ‘thyroid-like’ histology are increasingly being recognized at various sites such as kidney,4 oral cavity3 and minor salivary glands, and breast.5 Moreover, the probability of thyroid carcinoma arising in struma ovarii (monodermal teratoma) is also known.9 However, new to this expanding list of ‘thyroid-like’ carcinomas is a well-differentiated variant of intra-hepatic cholangiocarcinoma. Cholangiocarcinoma is the third most common malignant neoplasm of liver. It is usually not associated with liver cirrhosis. Many morphological patterns of cholangiocarcinoma have been described including tubular, mucinous adenosquamous, clear cells, sarcomatoid, lymphoepithelioma-like, etc.10 However, the most unusual variant is the ‘thyroid-like’ variant of cholangiocarcinoma, of which only three anecdotal reports have been published till date.6-8 Of these three cases, two arose in young women of ages 267 and 27 years,8 the index case also being a 24 year old woman, and the third reported case was in a 52 year old gentleman.6 This is in sharp contrast to the mean age of 74 years for patients of cholangiocarcinoma, with a distinct male preponderance, as documented in the SEER data.11 This variant’s tendency to affect young females in the third decade, analogous to papillary carcinoma of thyroid, is a notable finding. Liver involvement was seen in the form of isolated left lobe involvement,7 both left and right lobe involvement,8 and isolated right lobe involvement,6 in the previously reported cases. The index case had a large left lobe mass. Two out of three reported cases and the index case did not show any significant abnormalities in the surrounding liver parenchyma; one of the published cases had cirrhosis in the surrounding liver of cryptogenic origin.7
On microscopy, all the cases (including the present case) showed a prominent follicular architecture, resembling thyroid neoplasm with a combination of macro- and micro-follicles with colloid-like material. Furthermore, optically clear nuclei were seen in all the cases, including the index case. The relevant clinic-pathological characteristics of all the reported cases and the current case are given in Table 1. The immunohistochemical profile was also similar in all the cases, with absence of TTF-1 and thyroglobulin (thyroid-related markers, Fig. 2), and positivity for CK7, and CK19 (markers of cholangiocarcinoma in absence of thyroid-specific markers). In addition, PAX-8 (a transcription factor expressed in neoplastic and non- neoplastic thyroid tissue) was also negative in our case. Uniquely, in our case, carcinoma in the thyroid raised a very strong suspicion of a primary in thyroid with metastasis to the liver. However, the incidentally detected micro-carcinoma in thyroid with features of follicular variant of papillary thyroid carcinoma was immunohistochemically quite distinct from the liver tumor. All thyroid related markers (TTF-1, PAX-8, thyroglobulin) were diffusely positive in the thyroid tumor while being completely negative in the hepatic tumor (Fig. 2). Sub-centimeter thyroid primary with a very large hepatic metastasis was also an unrealistic probability given that occurrence of distant metastasis in thyroid microcarcinoma is an exceptionally rare event (<1%).12 In an uncommon clinical situation of synchronous tumors, IHC can easily ascertain the tissue of origin and rule-out/rule-in metastasis. In the present case, IHC helped to avoid erroneous classification of both resectable primaries into a palliative, unresectable category.
Biology of this newly recognized variant of intrahepatic cholangiocarcinoma is still unclear. Follow up was available in two previously reported cases. In the case reported by Foucar et al.,8 the tumor was inoperable at presentation, however the patient lived for 14 years after diagnosis, though eventually died of a tumor related complication. Fornelli et al.6 reported a disease-free follow up of 13 months post-surgery. In the present case, patient is asymptomatic 18 months after left hepatectomy. Though the number of cases is not sufficient to draw a conclusive interpretation, the findings hint towards a more favorable /indolent biology compared to conventional cholangiocarcinoma.
In conclusion, we report the fourth case of thyroid-like intrahepatic cholangiocarcinoma and a first with a synchronous follicular variant of papillary thyroid carcinoma. Young age, female proclivity, large mass at presentation and thyroid-like histology point towards a unique subset of intrahepatic cholangiocarcinoma that appear to have a relatively favorable biology. There is a need to recognize this rare variant in order to accumulate data and better understand of its biology and clinical behavior. Synchronous occurrence with a FVPTC is a challenging scenario that can simulate metastatic disease and mislead subsequent patient management. Whether demographic and morphologic similarity points to an underlying genetic link between the two different tumors needs exploration.
REFERENCES
1. Hapke MR, Dehner LP. 1979; The optically clear nucleus. A reliable sign of papillary carcinoma of the thyroid? Am J Surg Pathol. 3:31–38. DOI: 10.1097/00000478-197902000-00004. PMID: 534382.
2. DeLellis RA. 1993; Orphan Annie eye nuclei: a historical note. Am J Surg Pathol. 17:1067–1068. DOI: 10.1097/00000478-199310000-00014. PMID: 8372945.
3. Michal M, Kacerovska D, Kazakov DV. 2013; Cribriform adenocarcinoma of the tongue and minor salivary glands: a review. Head Neck Pathol. 7 Suppl 1(Suppl 1):S3–S11. DOI: 10.1007/s12105-013-0457-9. PMID: 23821209. PMCID: PMC3712093.

4. Amin MB, Gupta R, Ondrej H, McKenney JK, Michal M, Young AN, et al. 2009; Primary thyroid-like follicular carcinoma of the kidney: report of 6 cases of a histologically distinctive adult renal epithelial neoplasm. Am J Surg Pathol. 33:393–400. DOI: 10.1097/PAS.0b013e31818cb8f5. PMID: 19047894.
5. Chang SY, Fleiszer DM, Mesurolle B, El Khoury M, Omeroglu A. 2009; Breast tumor resembling the tall cell variant of papillary thyroid carcinoma. Breast J. 15:531–535. DOI: 10.1111/j.1524-4741.2009.00773.x. PMID: 19594763.

6. Fornelli A, Bondi A, Jovine E, Eusebi V. 2010; Intrahepatic cholangiocarcinoma resembling a thyroid follicular neoplasm. Virchows Arch. 456:339–342. DOI: 10.1007/s00428-009-0874-z. PMID: 20082203.

7. Chablé-Montero F, Shah A, Angeles- Ángeles A, Henson DE, Albores-Saavedra J. , Montante-Montes de Oca D. 2012; Thyroid-like cholangiocarcinoma of the liver: an unusual morphologic variant with follicular, trabecular and insular patterns. Ann Hepatol. 11:961–965. DOI: 10.1016/S1665-2681(19)31427-9. PMID: 23109464.

8. Foucar E, Kaplan LR, Gold JH, Kiang DT, Sibley RK, Bosl G. 1979; Well-differentiated peripheral cholangiocarcinoma with an unusual clinical course. Gastroenterology. 77:347–353. DOI: 10.1016/0016-5085(79)90291-9. PMID: 221305.

9. Barrera JR, Manalo LA, Ang FL. 2012; Papillary thyroid-type carcinoma arising from struma ovarii. BMJ Case Rep. 2012:bcr0320126145. DOI: 10.1136/bcr.03.2012.6145. PMID: 22787184. PMCID: PMC3417009.

10. Shaib Y, El-Serag HB. 2004; The epidemiology of cholangiocarcinoma. Semin Liver Dis. 24:115–125. DOI: 10.1055/s-2004-828889. PMID: 15192785.

11. Saha SK, Zhu AX, Fuchs CS, Brooks GA. 2016; Forty‐year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 21:594–599. DOI: 10.1634/theoncologist.2015-0446. PMID: 27000463. PMCID: PMC4861366.

12. Roti E, degli Uberti EC, Bondanelli M, Braverman LE. 2008; Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol. 159:659–673. DOI: 10.1530/EJE-07-0896. PMID: 18713843.

Fig. 1
Thyroid-like cholangiocarcinoma (A-C, upper panel): (A) Tumor displays a conspicuous macrofollicular pattern at low magnification. (B) Eosinophilic colloid-like secretions in the neoplastic follicles. (C) Microfollicular architecture with prominence of “Orphan Annie eye” nuclei. Follicular variant of papillary thyroid carcinoma (D-F, lower panel): (D) Tumor nodule separated from surrounding thyroid follicles by a thin capsule. (E) Macrofollicular pattern of the thyroid tumor with eosinophilic secretions in the lumen, similar to areas seen in the liver tumor, (F) Subtle nuclear grooving and focal clearing in the thyroid tumor, characteristic of Follicular variant of papillary thyroid carcinoma.
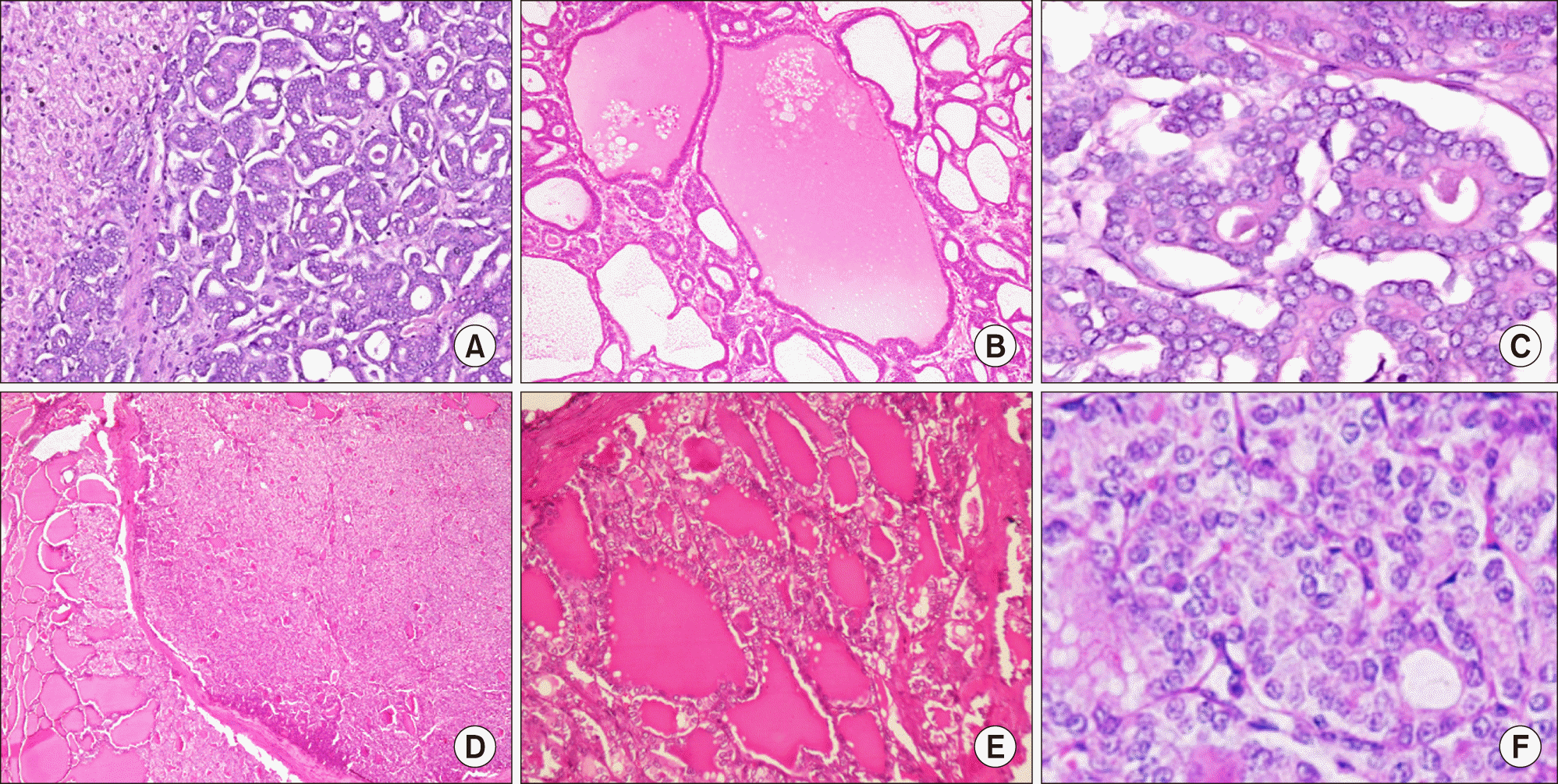
Fig. 2
Immunohistochemical profile of thyroid-like cholangiocarcinoma (A-D, upper panel) vis a vis papillary thyroid microcarcinoma (E-H, lower panel). (A) Hematoxylin & Eosin stained section of liver tumour showing microfollicular pattern. On immunohistochemistry, tumor showed strong positivity for CK7 (B) and focal for CK19 (not shown here). Intrahepatic cholangiocarcinoma is negative for thyroid specific markers, i.e. TTF-1 (C), and Thyroglobulin (D). Immunohistochemical profile of thyroid carcinoma (E-H, lower panel). (E) Thyroid tumor is encapsulated and has a microfollicular pattern. The tumor is diffusely positive for thyroid related markers, namely PAX8 (F), TTF-1 (G), and thyroglobulin (H).
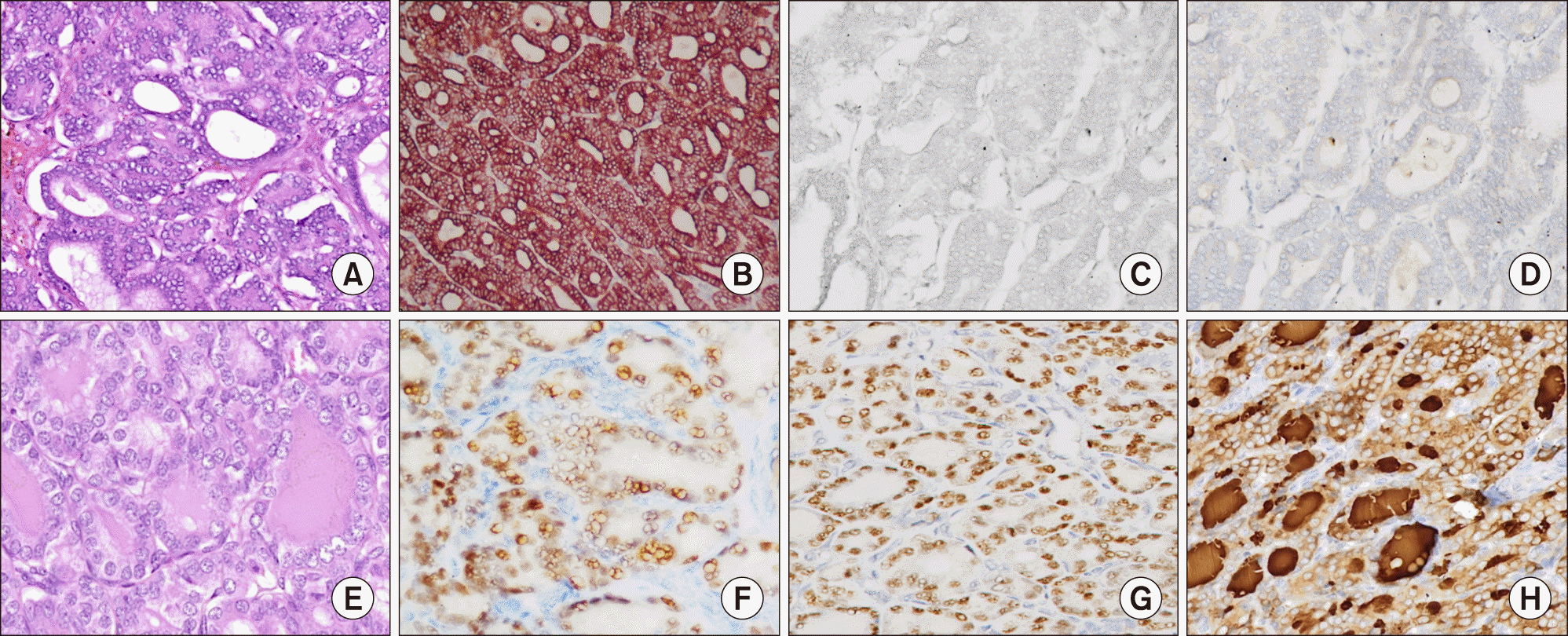
Table 1
Clinic-pathological characteristics of cases reported till date
| Clinico-pathological features | Chablé-Montero et al.7 | Fornelli et al.6 | Foucar et al.8 | Present case |
|---|---|---|---|---|
| Age/Sex | 26/F | 52/M | 27/F | 23/F |
| Transaminases | Normal | Not mentioned | Elevated Alkaline phosphatase, normal AST | |
| Viral markers | Non-reactive | Not mentioned | Not performed, orcein stain for Hepatitis B was negative | Non-reactive |
| Tumour size (largest dimension in cm) | 16 | 18 cm | Not mentioned | 12.1 cm |
| Gross findings | Encapsulated, multinodular, grey white with cytic and haemorrhagic areas | Well demarcated, with a brownish cut surface, multiple cystic cavities giving a spongy appearance | Firm tan-white tumour, mainly solid with few large cysts (post-mortem findings) | Solid-cystic with areas of haemorrhage, and a spongy architecture |
| Encapsulation | Yes, thick fibrous capsule | Yes, thin capsule | Yes, thick capsule | Yes, thick fibrous capsule |
| Microscopic features -pattern | Mixed Macro- and microfollicular, focal solid trabecular and insular | Mixed Macro- and microfollicular pattern | Small and large ductular structures, solid areas and small cell histology in metastatic locations | Mixed Macro- and microfollicular pattern |
| Luminal secretions | Yes, colloid like | Yes, colloid like | Yes, amorphous material positive for PAS | Yes, colloid like, and PAS positive |
| Papillary-like nuclear features | Yes, nuclear clearing with occasional grooves | Yes, nuclear clearing with occasional grooves | Oval nuclei with finely granular chromatin | Yes, nuclear clearing with occasional grooves |
| Mitosis | In insular areas, count not mentioned | 1/10 hpf | Numerous in the solid areas | 1/10 hpf |
| Necrosis | No | No | Yes, in areas with small cell histology | No |
| Angioinvasion | Yes | No | Yes | No |
| Sorrounding liver parenchyma | Non-cirrhotic at diagnosis, later developed cryptogenic cirrhosis | Mild steatosis, non-cirrhotic | Mild portal fibrosis, non-cirrhotic | Non-cirrhotic, mild portal fibrosis |
| Metastasis (time to metastasis) | Adrenal glands and regional nodes (18 months) | No | Lungs (180 months) | No |
| Thyroid gland (mode of investigation) | Unremarkable (Clinical, USG and CT scan) | Unremarkable (Clinical, USG and CT scan) | Not evaluated | Follicular variant of Papillary thyroid carcinoma (USG neck) |
| Treatment | Left Hepatic lobectomy followed by adjuvant chemotherapy | Right hepatic lobectomy | No treatment, autopsy done post-mortem | Left hepatic lobectomy |
| Follow up in months | 18 | 13 | 180 | 36 |
| Status at last follow up | Dead of disease | No evidence of disease | Dead of disease | No evidence of disease |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download