I. Introduction
Malignant fibrous histiocytoma (MFH) was first described by Ozzello et al.
1
in 1963 and by O’Brien and Stout
2
in 1964. It exhibits a fibroblastic/myofibroblastic nature with facultative histiocytic differentiation and was once considered the most common soft tissue sarcoma in adults. However, with the advancement of diagnostic and imaging approaches, many of these previously diagnosed MFHs have been reclassified as a more specific entity based on their lineage of differentiation
2-4.
More recently, the World Health Organization classification of soft tissue tumors has modified the term “MFH” to include undifferentiated pleomorphic sarcoma (UPS), which is now the preferred term in the literature and fundamentally represents a diagnosis of exclusion
5
. Clinically, this malignant tumor preferentially affects deep soft tissues of the extremities (mainly the lower limbs) of older patients during their sixth to seventh decades of life, with a rapidly progressive growth pattern
5
. Its aggressiveness is also demonstrated by the high frequency of metastases that are identified when the primary tumor is diagnosed
4
.
UPS that has infiltrated the bone is very uncommon, and involvement of the gnathic bones is even rarer. Only a few case reports currently available in the literature have documented this neoplasm in the oral cavity
4-6. Therefore, not only is its microscopic presentation complex, but its rarity makes an oral UPS diagnosis a challenge for diagnosticians. Report of new cases may contribute to a better understanding of its clinical and radiographic features. Therefore, in this manuscript, we aimed to describe an original case of UPS in the mandible of an 88-year-old female patient.
II. Case Report
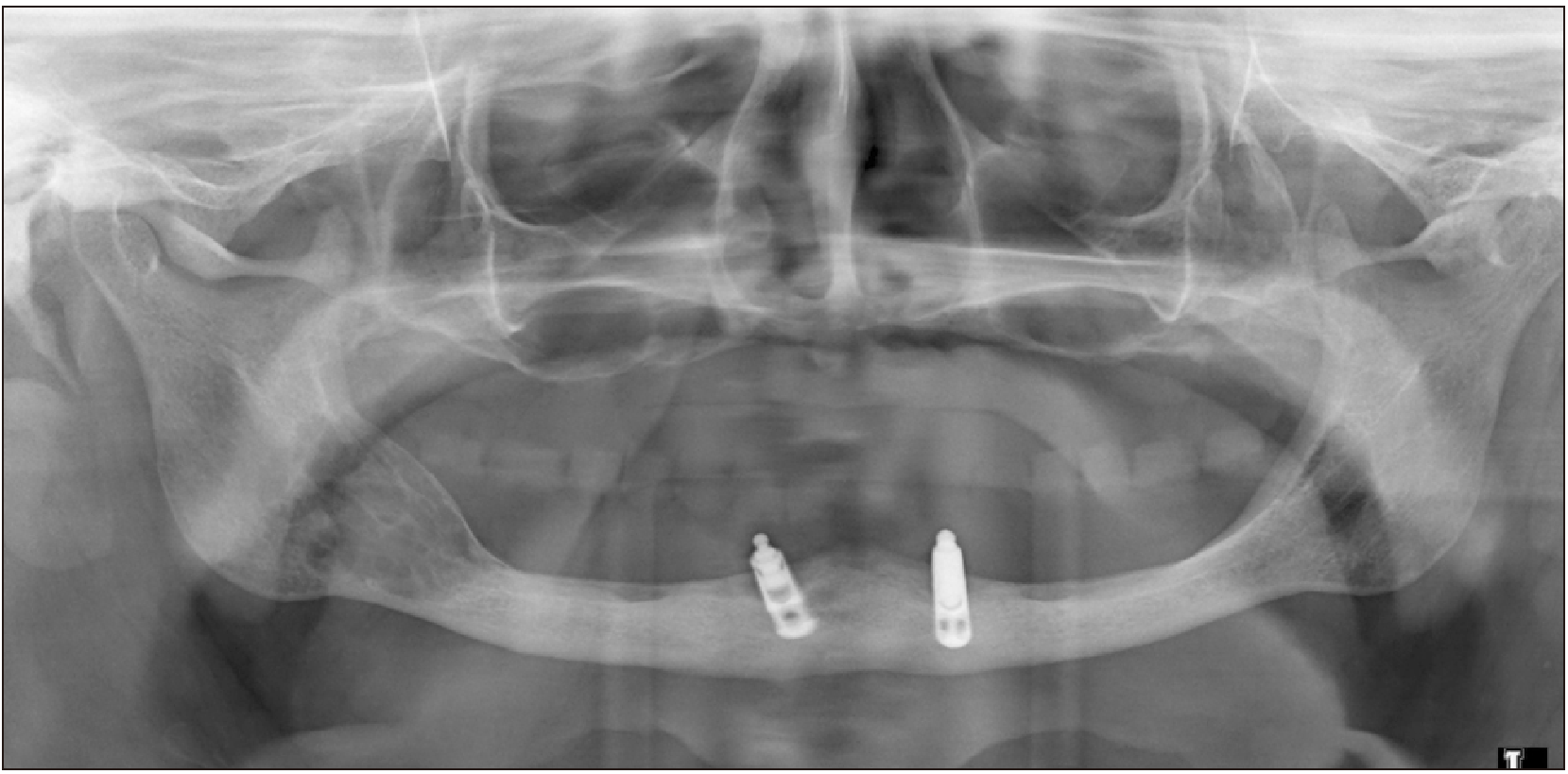
An 88-year-old female patient was referred to our department chiefly complaining of a mandibular swelling. The patient was a non-smoker and non-drinker, with a positive history of glucose intolerance. During anamnesis, the patient reported that she had a previous diagnosis of a central giant cell lesion affecting the posterior mandible of the right side that was imaged in 2015.(
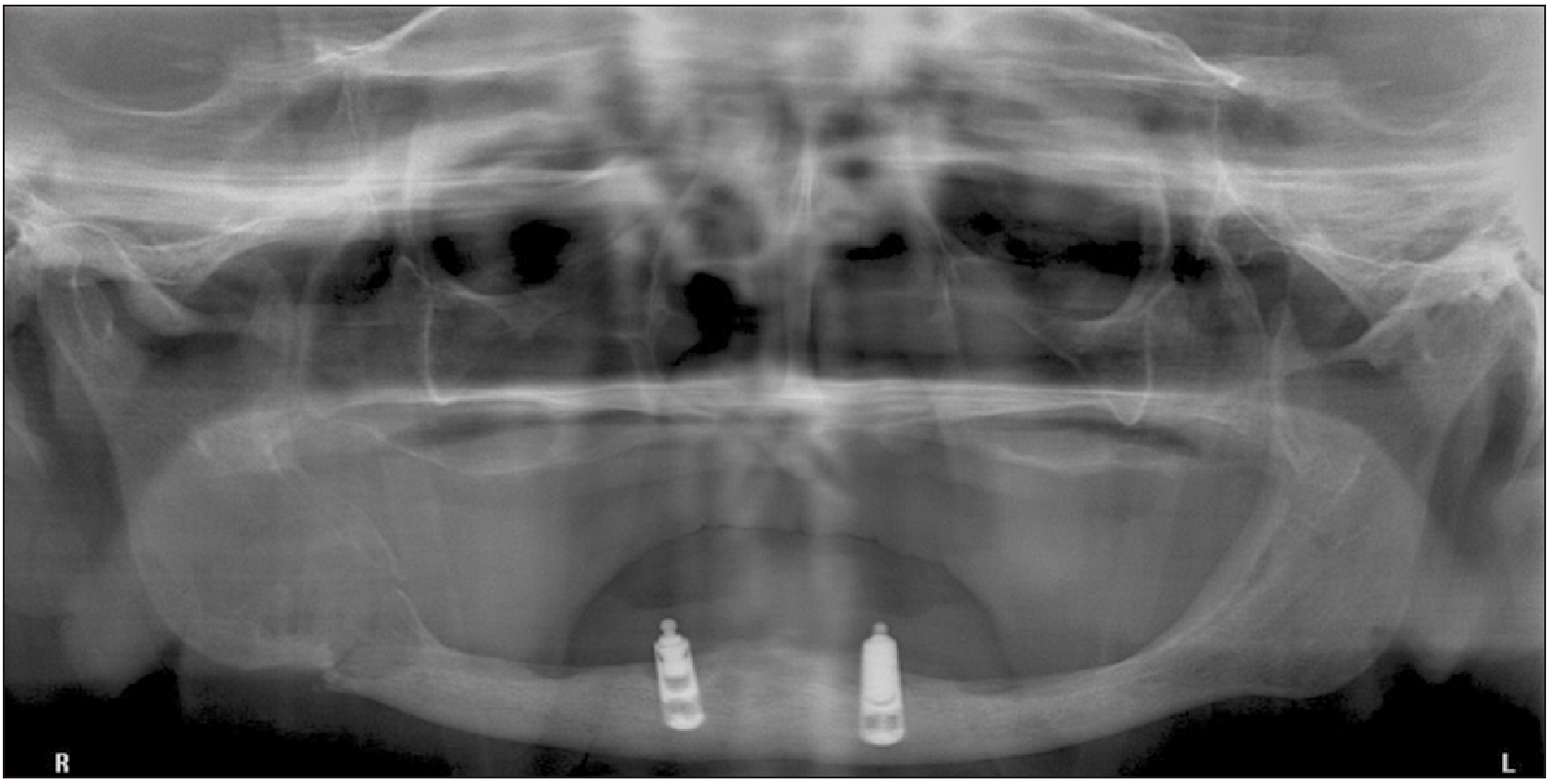
Fig. 1) Five months after the first incisional biopsy, the patient demonstrated persistent strong pain and continuous growth of the lesion. A new radiographic exam revealed a radiolucent image with ill-defined, irregular borders and a pathological fracture on the right side of the mandible.(
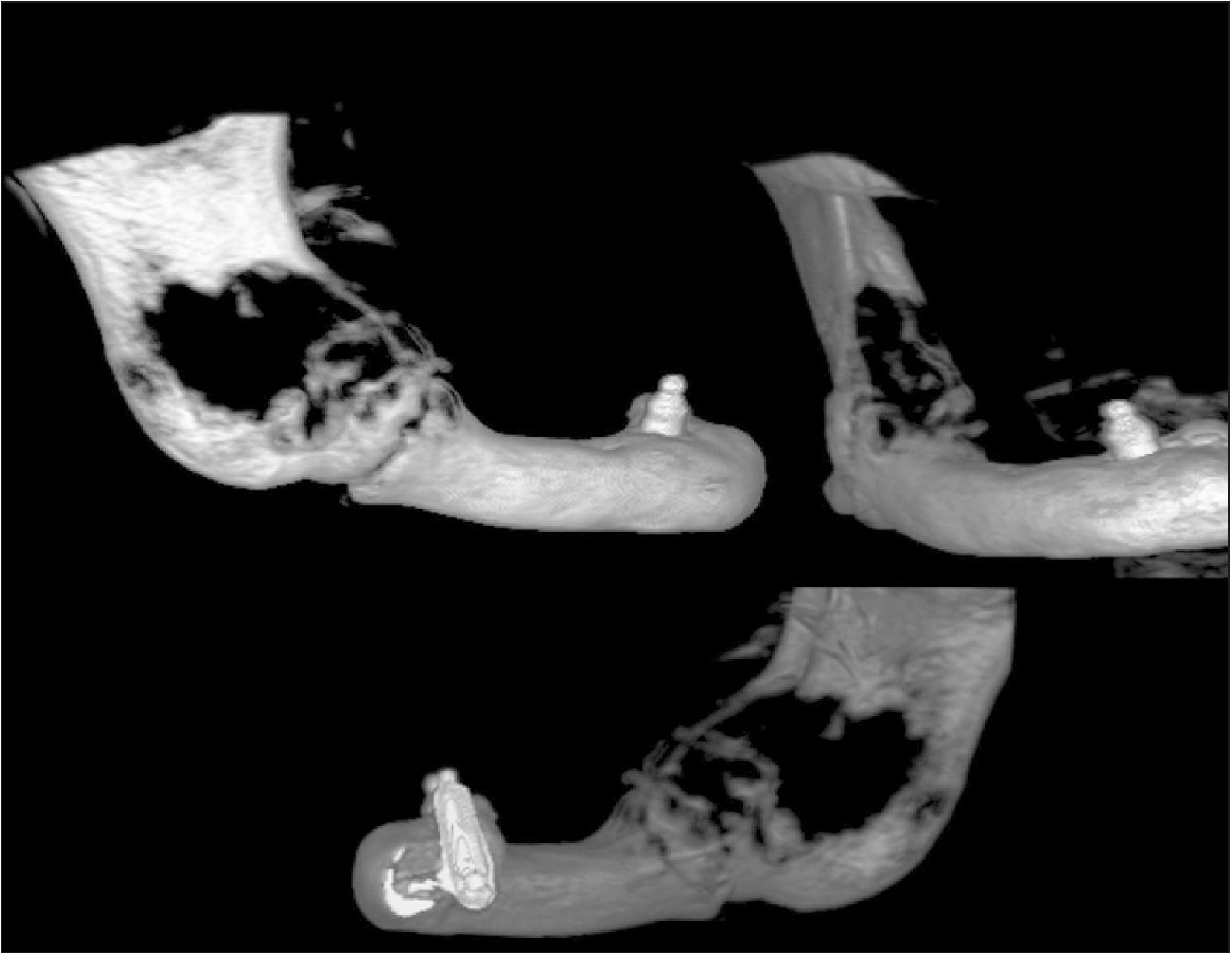
Fig. 2) The computed tomography scan with tridimensional reconstruction showed that the bone lesion involved half of the mandibular body and three-quarters of the ascending ramus.(
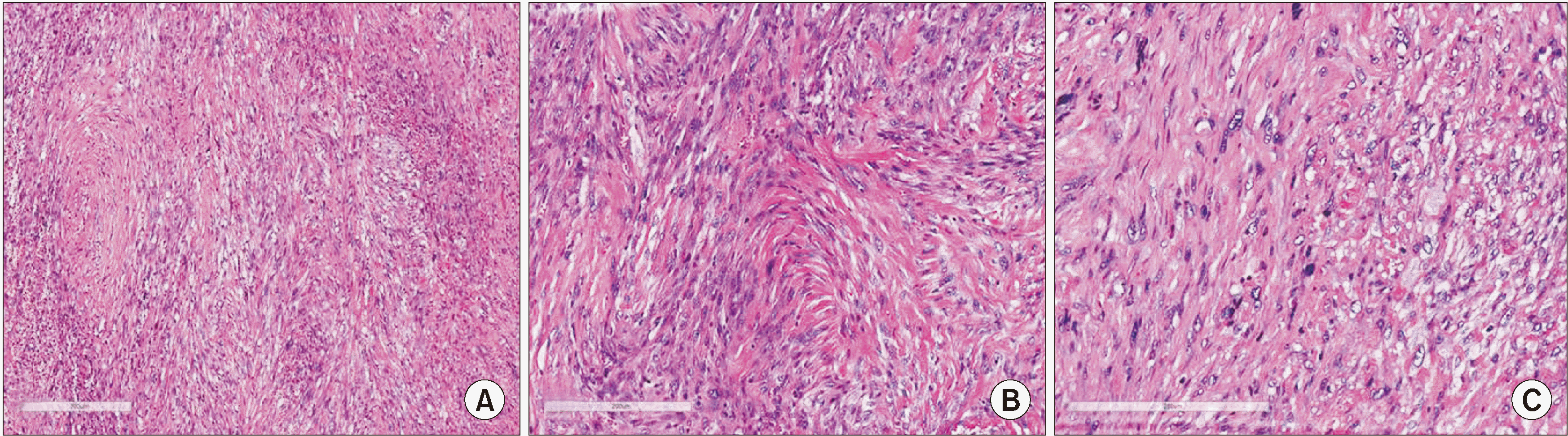
Fig. 3) A new biopsy was performed, and the microscopic evaluation revealed the presence of an infiltrative, undifferentiated spindle-cell neoplasm with moderate to severe cellular pleomorphism, atypical mitotic figures, and areas of tissue necrosis with no evidence of osteoid production.(
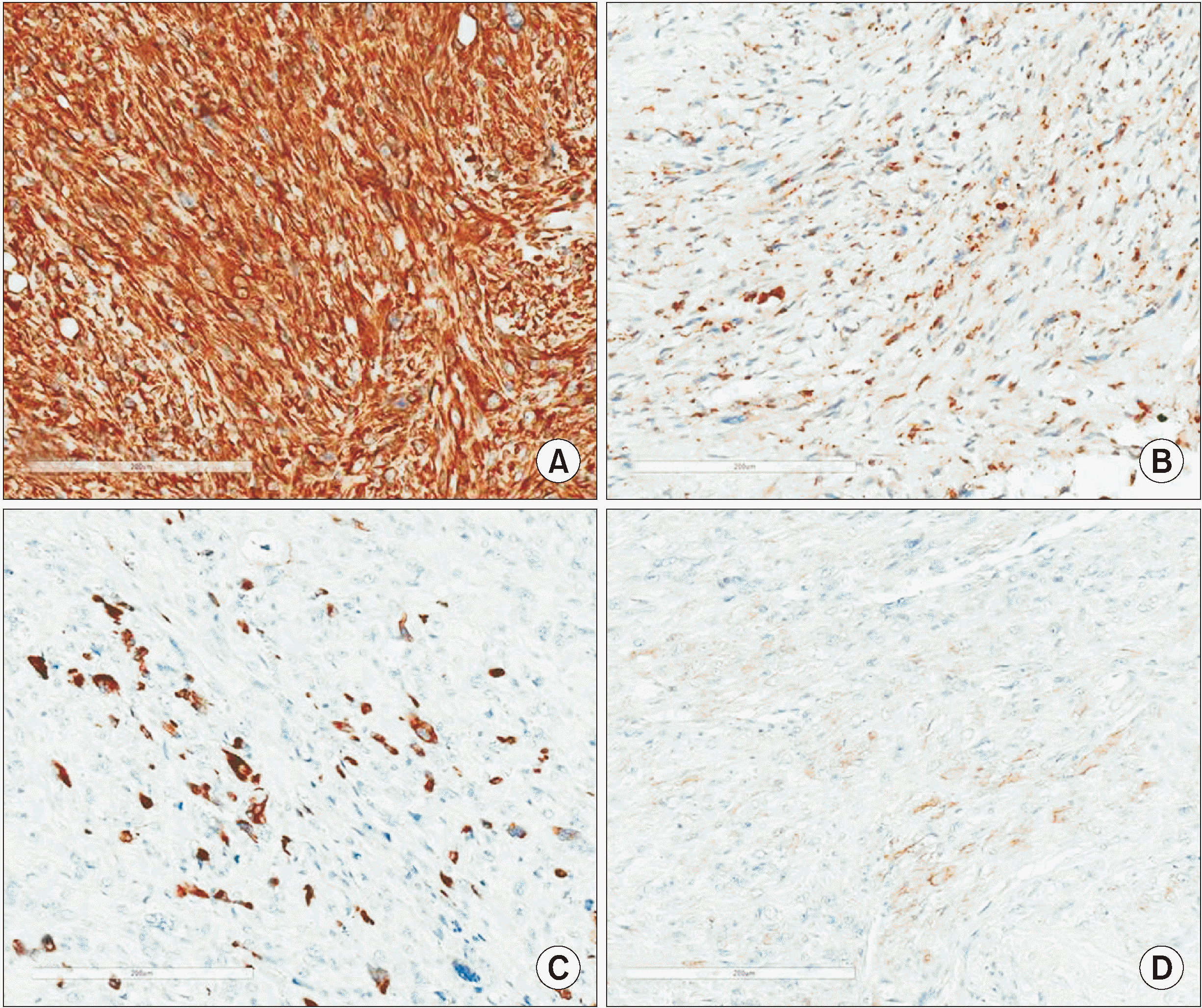
Fig. 4) Immunohistochemistry was carried out and was negative for beta-catenin, h-caldesmon, CD34, epithelial membrane antigen (EMA), desmin, and S100; strongly positive for vimentin; and only focally positive for smooth muscle actin (SMA), cytokeratin (AE1/AE3), and CD68 (
Fig. 5), leading us to a final diagnosis of UPS.
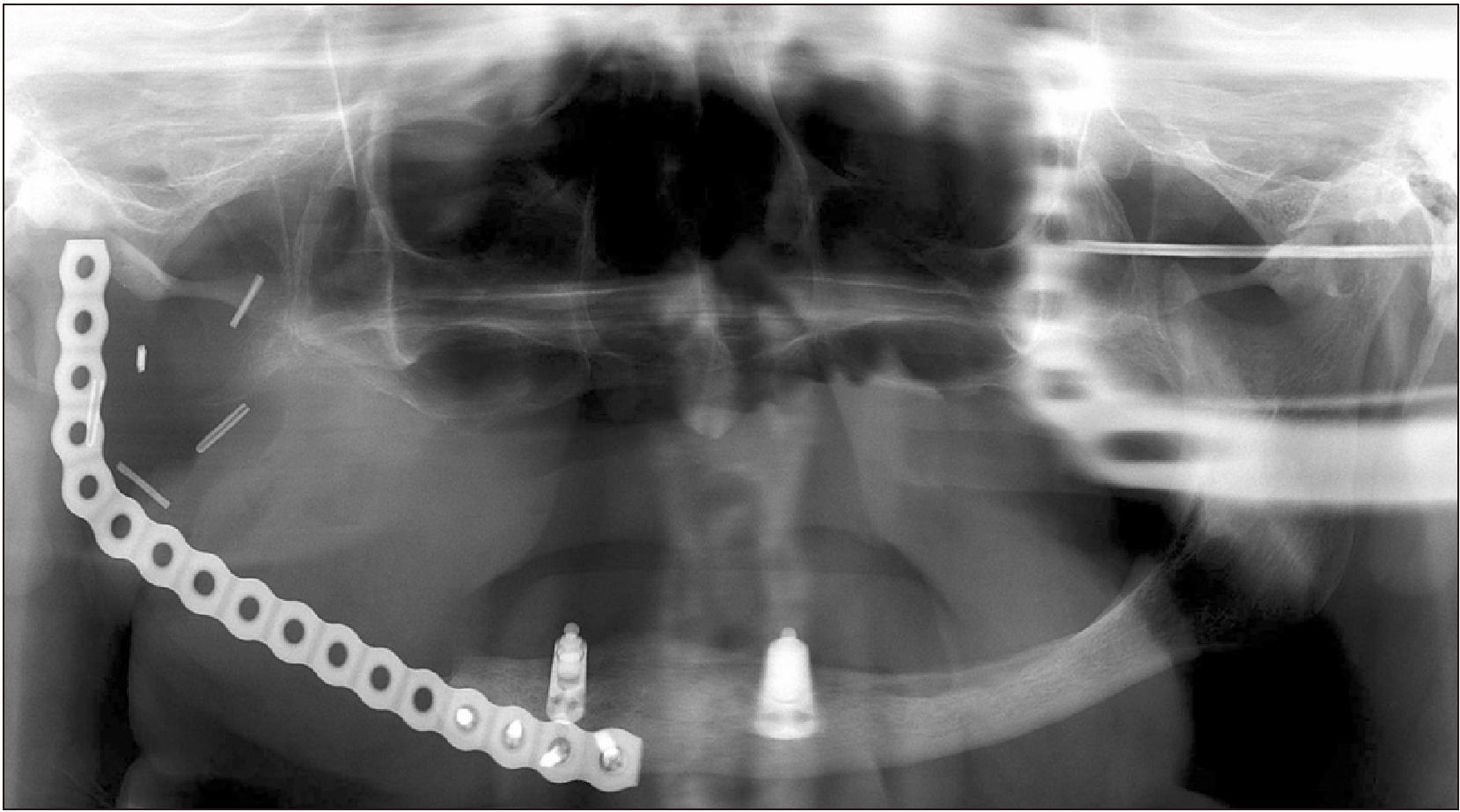
The patient underwent right segmental mandibulectomy with condyle disarticulation, right subtotal parotidectomy, and cervical neck dissection. Microscopic analysis of the surgical specimen revealed perineural, muscular, and adipocytic invasions, with positive margins adjacent to the anterior gingival tissue, larynx, floor of the mouth, and retromolar region. The histological aspect of the neoplasm suggested an aggressive spindle-cell neoplasm with multiple multinucleated giant cells. Immunohistochemistry was applied to the surgical specimen and demonstrated diffuse staining for vimentin and CD68 and only focal reactivity for desmin, SMA, and HHF-35; no positivity was found for AE1/AE3, S-100, or CD34 and h-caldesmon, confirming the diagnosis of UPS.
The patient received a cycle of 60-Gy (30 sections of 2 Gy) radiotherapy followed by an additional boost cycle of 6 Gy (3 sections of 2 Gy) for a total of 66 Gy. During anti-neoplastic therapy, the patient exhibited Grade I mucositis, dygeusia, Grade I xerostomy, and Grade II radiodermy. After six months of follow-up, the patient was well, with no evidence of local recurrence and under strict follow-up.(
Fig. 6) Unfortunately, 12 months after surgery, she presented with lung metastasis and died.
III. Discussion
UPS was once believed to account for up to 40% of adult soft tissue sarcomas
3
. However, it is now considered a much less common entity; immunohistochemistry and molecular biology assays indicate that most previously designated MFHs actually represent pleomorphic leiomyosarcomas, rhabdomyosarcomas, liposarcomas, osteosarcomas, malignant nerve sheath tumors, melanomas, lymphomas, and carcinomas. UPS that affects the oral cavity and the gnathic bones is very rare; only a few cases have been described to date: myofibroblastic sarcoma
7
, low-grade myxofibrosarcoma
8
, sarcomatoid carcinoma
9
, osteogenic sarcoma
10
, Ewing’s sarcoma
11
, osteosarcoma
12
, and odontogenic carcinosarcoma
13
, all involving the mandible. Here, we describe an additional case that will be useful in understanding the clinical and radiographic presentation of the condition.
UPS is a very aggressive neoplasm that predominantly affects the upper and lower extremities and the retroperitoneum; only 10% of cases are diagnosed in the head and neck area and account for less than 0.5% of all malignant neoplasms located in this region
3,14
. The etiology of UPS is largely unknown, but some cases are thought to originate following radiation therapy
3,15
. To be considered a post-radiation neoplasm, the tumor must be located within the radiation field and must develop at least three years after radiation therapy in an area that was free of tumors prior to radiation
16
; there is an estimated lifetime risk of up to 0.3%
17
. In addition, some authors have also suggested that UPS may develop from its formerly believed benign counterpart, benign fibrous histiocytoma
17
. The literature shows that radiation-induced sarcoma development may be influenced by factors including radiation dose, patient age at initial exposure, prior exposure to chemotherapeutic agents, and a genetic tendency
17
.
As demonstrated in the current report, UPS usually develops in older adults in their fifth to seventh decades of life, and males are affected more often than females
18
. Our review (
Table 1)
4,5,8,11,18-34 identified a mean age of 50.5 years at the diagnosis and a standard deviation of 19.7 years; 21% of patients who undergo radiation treatment eventually develop UPS. Symptoms usually depend on the anatomical site involved, but the neoplasm usually presents as a rapid growth with or without pain
16
. Our review found that only 30% of patients complained of pain, while 42% sought professional help due to swelling. In the head and neck, the sinonasal tract was the most commonly affected site
19
, which could potentially lead to dysphagia, pain, and nasal obstruction. In the oral cavity, the mandible was involved in 70% of cases. UPS commonly manifests as diffuse, ill-defined, painful swellings that typically affect the mandible and cause extensive bone destruction that is associated with irregular radiolucent uni- or multilocular images and may also be associated with floating teeth depending on tumor size
18,19
. The neoplasm may also cause pathological fractures
14
, as demonstrated in our case.
Histogenesis of UPS remains debatable; in contrast to the original concept, tumors do not seem to have a true histiocytic differentiation and instead are more likely to harbor a fibroblastic/myofibroblastic nature
5
; therefore, the term “MFH” will likely be completely discontinued in future publications. UPS is a diagnosis of exclusion when no other more specific entity is identified after extensive histological examination and use of ancillary diagnostic techniques. Microscopically, UPS is a heterogeneous malignancy with a variable cellular presentation and a predominantly fibrous stroma. All cases share a marked cytological and nuclear pleomorphism, with atypical mitosis and areas of necrosis, a predominant spindle-cell component, and scattered round histiocyte-like cells. A storiform growth pattern is recognized in most cases. Some cases may reveal extensive inflammatory infiltrate and presence of an evident multinucleated giant cell component
18,19
. We believe that this microscopic finding along with inadequate sampling of the lesion led to the initial misdiagnosis of a central giant cell lesion in the present case.
Immunohistochemistry is of great importance in achieving a final diagnosis. UPS usually demonstrates strong positivity to vimentin and variable reactivity to the histiocytic marker CD68. However, as illustrated in the present report, focal reactivity to smooth muscle actin (α-SMA), muscle specific actin (HHF-35), and desmin is occasionally observed. However, such findings should not be interpreted as true myogenic differentiation and instead more likely represent a myofibroblastic component, which is further supported by negativity to h-caldesmon
16
. Focal aberrant expression of cytokeratin may also be observed, as seen in this case. Reactions against S100 and CD34 must also be carried out to exclude melanoma and vascular/perivascular neoplasms, respectively.
The majority of UPS cases we reviewed (73%) included an incisional biopsy, but the clinician selected fine-needle aspiration for diagnosis in 9%. The patients underwent surgery without any evidence of a biopsy (data not shown) in 12% of reviewed case reports. This approach is not the best method of diagnostic management and should be discouraged.
Treatment of the affected patients involves complete surgical removal of the neoplasm and was the definitive treatment in 64% of our reviewed cases. Since regional metastases are only observed in approximately 15% of patients
14,18
and were uncommon in the cases we reviewed (
Table 1), neck dissections are not advocated in most cases, whereas adjuvant radiotherapy is preferentially recommended in unressectable cases or in those where clear margins cannot be achieved
14,18
. In the present report, the patient underwent segmental mandibulectomy and total removal of the tumor; however, because of the presence of involved surgical margins, radiotherapy was also administered. Because the initial scheme of 60 Gy (30×2 Gy) was well-tolerated, an additional boost of 6 Gy (3×2 Gy) was additionally recommended. The literature showed that 18% of UPS cases in bone were treated with surgery and adjuvant radiotherapy.
Recurrences are common, and distant metastases are likely to arise in the lung, liver, and bone, as observed in our patient. Some authors report that 25% to 35% of patients with UPS in the head and neck region will develop metastases. Background (primary or recurrent), tumor depth, size, and stage are strongly associated with prognosis; survival rates vary from 30% to 74% after five years of follow-up
3,4,16
irrespective of the type of treatment applied for UPS
18
. Our review demonstrated an average of 40.7 months of follow-up in living patients; after 12 months, 42% of the patients had died.(
Table 1) However, aggressive surgical management to achieve clear margins, particularly a minimum of 3-cm tumor-free margins
18
, is integral to effective treatment of UPS because overall median and five-year survival rates increase with clear surgical margins
14
. In addition, infiltrative growth behavior, as was seen in our case, is an adverse prognostic factor not only for local control, but also for disease-free and metastasis-free survival
14,18
.
UPS is an aggressive malignant neoplasm that rarely involves the oral cavity and gnathic bones, and that demands an extensive diagnostic effort to exclude other lineage-specific neoplasms. Therefore, diagnosticians must be aware of this entity when dealing with aggressive tumors that destroy the soft and hard tissues of older patients and also those who have previously undergone radiotherapy.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download