Abstract
Objectives
Materials and Methods
Notes
Authors' Contributions
S.S. and M.A. participated in data collection and coordination and wrote the manuscript. A.A., S.S., and M.A. participated in the study design. A.Y. is the supervision of the study and he read and approved the final manuscript. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
Experiments were carried out in accordance with guidelines from the European Community Council Directive of 24 November 1986 (86/609/EEC) and the Ethics Committee of Islamic Azad University, Khorasgan Branch approved the study (IR.IAU.KHUISF.REC.1397.262).
How to cite this article: Yadegari A, Aminzadeh A, Seyyedkhamesi S, Aminian M. The effect of melatonin on prevention of bisphosphonate-related osteonecrosis of the jaw: an animal study in rats. J Korean Assoc Oral Maxillofac Surg 2020;46:266-274. https://doi.org/10.5125/jkaoms.2020.46.4.266
References
Fig. 1
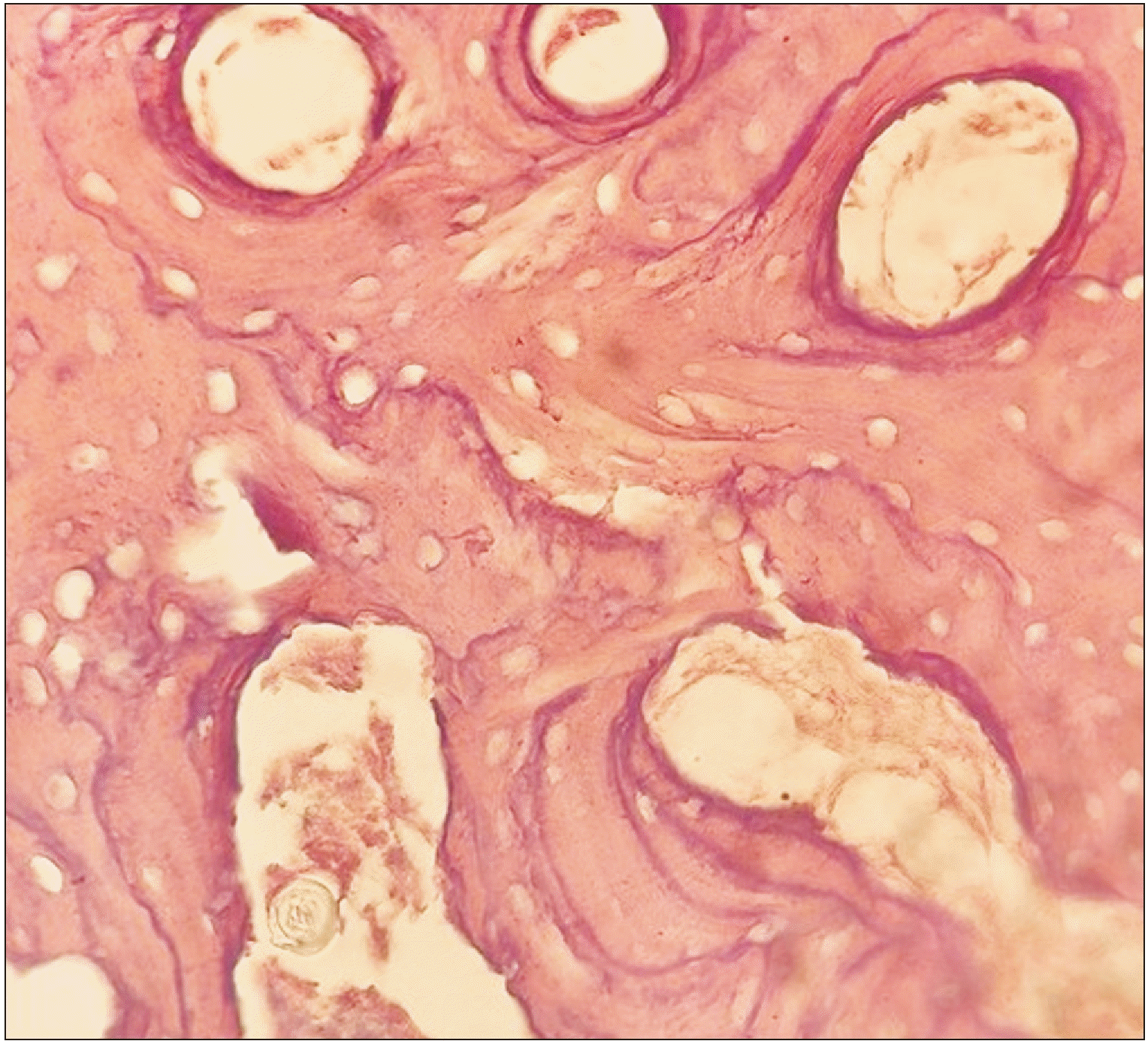
Fig. 2
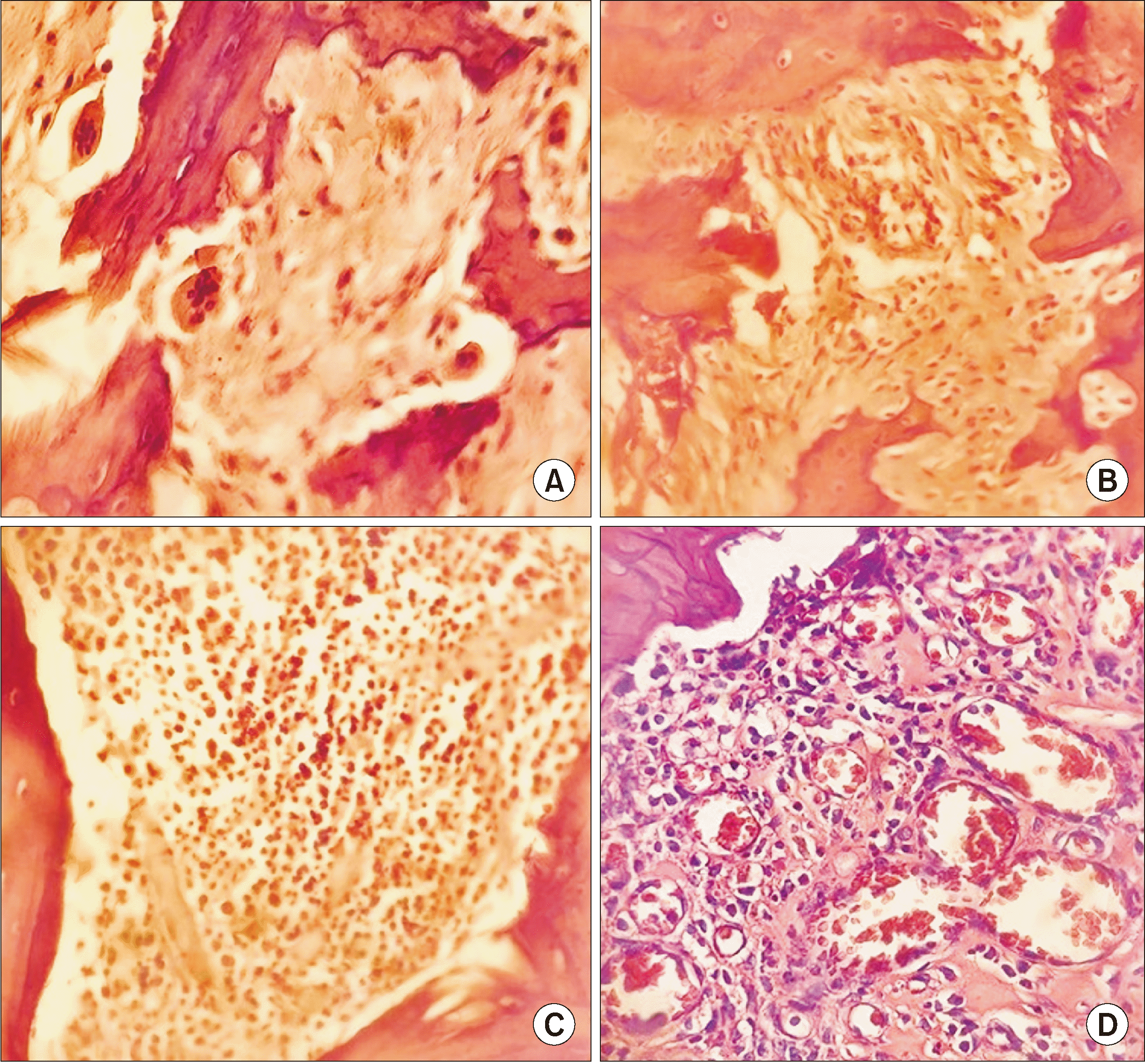
Table 1
| Factor | Study | Year | Country | Conclusion |
|---|---|---|---|---|
| Estrogen | Vaszilko et al.8 | 2014 | Hungary | Breast cancer patients had a significantly worse prognosis than patients with other underlying illnesses, which may indicate antiestrogen therapy was a causative factor. |
| Parathyroid hormone | Keskinruzgar et al.12 | 2016 | Turkey | Teriparatide was found to be effective in eliminating the negative effects of bisphosphonates on osteoclasts and the inflammatory phase of bone healing and had positive effects in preventing osteonecrosis. |
| Dayisoylu et al.13 | 2013 | Turkey | Administration of 30 μg/kg/day parathyroid hormone during a period of 8 weeks had positive effects on the resolution of BRONJ. | |
| Zandi et al.14 | 2017 | Iran | Four weeks of triparatide therapy, beginning at the same day or 2 weeks before tooth extraction, had a potential role in preventing osteonecrosis of the jaw. | |
| Autologous platelet concentrate (APC) | Del Fabbro et al.15 | 2015 | Italy | A review of results of 18 studies is suggestive of possible benefits of APC when associated with surgical procedures for treatment or prevention of BRONJ. |
| Mesenchymal stem cell (MSC) | Kaibuchi et al.3 | 2016 | Japan | Allogeneic MSC sheet transplantation is a promising alternative approach for treating BRONJ. |
| Ogata et al.16 | 2015 | Japan | The anti-apoptotic and anti-inflammatory effects of MSC dramatically regulated the turnover of local bone and indicated therapeutic effects on BRONJ. | |
| Antibiotic (penicillin) | López-Jornet et al.17 | 2011 | Spain | Antibiotic prophylaxis in invasive dental procedures results in a significant decrease in BRONJ. |
| Vitamin D | Yanık et al.18 | 2016 | Turkey | There is some evidence for the treatment of BRONJ with systemic use of vitamin D. |
| Geranylgeraniol | Koneski et al.19 | 2018 | Macedonia | Geranylgeraniol in a local solution form may be a promising option for prevention and treatment of BRONJ. |
Table 2
Table 3
| Variable | Group 1 (n=10) | Group 2 (n=10) | Group 3 (n=10) | P-value |
|---|---|---|---|---|
| Grade of fibroblasts | 0.006* | |||
| Grade 0 | 0 (0) | 2 (20.0) | 6 (60.0) | |
| Grade 1 | 7 (70.0) | 4 (40.0) | 4 (40.0) | |
| Grade 2 | 1 (10.0) | 4 (40.0) | 0 (0) | |
| Grade 3 | 2 (20.0) | 0 (0) | 0 (0) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download