I. Introduction
Full or partial edentulism in children and adolescents occurs most commonly as a result of trauma or genetic disorders, and these patients encounter esthetic and functional problems
1
. Esthetic problems can cause psychologic disorders and affect social activity
1,2
. Long-standing edentulism leads to a senile profile and a decreased facial height
3
. The functional problems experienced include difficulty in eating and speaking
1,2
. Papillon–Lefèvre syndrome (PLS; OMIM#245000) is one of the disorders that can cause early tooth loss
3,4
, and those affected by PLS usually need oral rehabilitation to overcome these problems
3
.
PLS is a rare autosomal recessive disorder with dermal and oral manifestations
4,5
. Mutation in the cathepsin C gene (CTSC), which is localized on chromosome 11q14-q21, in these patients causes the loss of cathepsin C function. Cathepsin C plays a role in epithelial differentiation and desquamation
6
. The severity of dermal manifestations, including diffuse hyperkeratosis affecting the palms of the hands and soles of the feet, varies in patients from mild psoriasiform scaly skin to overt hyperkeratosis
4,5
. The soles of the feet are usually affected more than the palms, and the degree of hyperkeratosis may be affected by seasonal changes. There is evidence that the hyperkeratosis severity is associated with periodontal involvement
5
. PLS patients’ neutrophil functions are affected, and these dysfunctions appear to be localized to tissues that are under direct and chronic bacterial attacks like the periodontal tissue. This has been suggested to be the possible reason for PLS patients’ lack of systemic infections
7
.
Rapid progressive destructive periodontitis around primary and permanent dentitions is the oral manifestation of PLS
8
. The inflammatory gingival changes and periodontitis around primary dentition appear concomitant with palmoplantar keratosis and subside after tooth loss
5
. Because of this, almost all PLS patients lose their teeth at a young age. Surprisingly, third molars, which erupt at older ages, are not affected
3,5
. PLS patients’ immune system function improves with age and their polymorphonuclear (PMN) cells’ chemotaxis and phagocytosis abilities become normal after some years of being edentulous
8,9
.
Dental implants can be a viable treatment option for these patients in order to overcome esthetic and functional prosthetic problems
10
. However, issues surrounding implantation raise success concerns in these patients; these issues include impaired patient immune systems and severe bone loss that results in the need for complex ridge augmentations
10,11
. The aim of this systematic review was to assess the clinical outcome and the survival rate of dental implants used for the oral rehabilitation of PLS patients.
Go to :

II. Materials and Methods
The present study is a systematic review that was performed in accordance with the “Preferred Reporting Items for Systematic Review and Meta-Analyses Protocols” (PRISMA-P, 2015)
12. The search was performed using “MeSH” terms and keywords based on the elements of the PICO question:
1. Participants (P): PLS patients
2. Intervention (I): Oral rehabilitation using dental implants
3. Comparison or control (C): Not applicable
4. Outcome measures (O): Success of dental implant
1. Information sources and search strategy
An exhaustive search of the literature available in PubMed Central, Scopus, and Web of Science’s electronic databases until July 2019 was conducted. The following keywords were used in the search strategy: “Papillon–Lefèvre syndrome” AND “dental implant” OR “prosthodontics”. The full title and abstract of each article were screened by two independent authors (F.A. and S.K.) using predetermined inclusion and exclusion criteria.(
Fig. 1) All of the references were selected from the EndNote X9 (Thomson Reuters, Philadelphia, PA, USA). In order to ascertain whether any relevant studies were neglected in the initial search, the bibliographies of the selected studies and Google Scholar were also reviewed. Any differences in the selection of the studies were resolved by discussion with a third reviewer (S.A.).
 | Fig. 1Diagram of literature search and selection criteria adapted from PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 12. (PLS: Papillon–Lefèvre syndrome) 
|
2. Eligibility criteria
Inclusion criteria were articles written in English with full text availability that reported on oral rehabilitation of PLS patients with dental implants, including, for example, case reports and case series.
Exclusion criteria were articles on other prosthetic rehabilitation methods besides dental implants or articles without treatment outcome.
3. Data collection
Data were extracted from the included articles by two independent authors (F.A. and S.K.). A third author resolved any disagreements in the extracted data. Microsoft Excel 2016 (Microsoft, Redmond, WA, USA) was used for standardization and organization of the extracted data.
The following information was collected (if available) and is provided in
Table 1:
Table 1
Data extracted from selected articles
|
Study |
Sex |
Age (yr)
1
|
Treatment modality |
Implant number |
Follow up (yr)
2
|
Outcome |
Probable reason |
|
Kinaia et al.20 (2017) |
M |
21 |
Rehabilitation of maxilla & mandible using calvarium bone graft
Implant placement (after 3 months)
Prosthetic rehabilitation (after 3 months) |
Max: 9
Man: 6 |
1 |
Healthy |
|
|
Nickles et al.11 (2013) |
|
|
|
|
|
|
|
|
Case 1 |
F |
20 |
Dental implant placement |
Max: 6
Man: 4 |
4 |
Incipient peri-implantitis |
Poor oral
Hygiene |
|
Case 2 |
F |
20 |
Dental implant placement |
Max: 6
Man: 4 |
2 |
Healthy |
|
|
Case 3 |
F |
18 |
Dental implant placement |
Max: 6
Man: 8 |
Max: 4
Man: 20 |
Peri-implantitis
Max: 4 implants lost |
Poor oral
Hygiene |
|
Case 4 |
F |
19 |
Dental implant placement |
Max: 10
Man: 10 |
Max: 8
Man: 10 |
Peri-implantitis
Max: 9 implants lost
Man: 6 implants lost |
Poor oral
Hygiene & compliance |
|
Rai et al.23 (2014) |
M |
26 |
Extraction of all teeth
Maxillary conventional denture
Mandibular implant supported overdenture (6 months after implant placement) |
Man: 2 |
1 |
Healthy |
|
|
Al Farraj AlDosari21 (2013) |
F |
19 |
Extraction of all teeth except second molars
Transitional lower partial denture
Dental implant insertion (after 2 months)
Simultaneous bone grafting with Bio-Oss in needed sites
Prosthetic rehabilitation (after 4 months)
Periodic follow-up |
Max: 8
Man: 6 |
1 |
Healthy |
|
|
Senel et al.16 (2012) |
M |
18 |
Saving two third molars
Implant placement and prosthetic rehabilitation (after 12 months)
Regular follow-up |
Max: 6
Man: 6 |
3 |
Max: 1 implant lost (early failure) |
Lack of osseointegration |
|
Ahmadian et al.10 (2011) |
F |
21 |
Complete denture (interim)
Mandibular set back (sagittal split ramus osteotomy)
Another mandibular denture (after 1 month)
Nerve repositioning for posterior mandible & simultaneous implant placement (after 9 months)
Bilateral open sinus augmentation (tibia bone graft)
Maxillary implant placement (after 9 months)
Vestibuloplasty for increasing attached gingiva & prosthetic rehabilitation (after 6 months) |
Max: 8
Man: 8 |
4 |
Healthy |
|
|
Etöz et al.17 (2010) |
F |
34 |
Implant-supported overdenture in mandible using short implants (6 mm in height and 4.1 mm in diameter at the canine regions)
Prosthetic rehabilitation (after 3 months) |
Man: 2 |
1 |
Healthy |
|
|
Toygar et al.3 (2007) |
F |
18 |
Alveolar bone augmentation (demineralized bone matrix and a titanium membrane)
Membrane removal (after 8 weeks)
Implant placement (after 3 months) and simultaneous repeated augmentation for bone thickening (esthetic requirement) |
Max: 2 |
NM |
Healthy |
|
|
Dhanrajani22 (2004) |
|
|
|
|
|
|
|
|
Case 1 |
M |
NM |
Fixed implant-retained mandibular prostheses |
Man: 6 |
9 |
Healthy |
|
|
Case 2 |
M |
NM |
Fixed implant-retained mandibular prostheses |
Man: 6 |
9 |
Healthy |
|
|
Woo et al.18 (2003) |
M |
14 |
Implant-retained overdenture 4 months after implant placement |
Man: 2 |
1 |
Healthy |
|
|
Ullbro et al.8 (2000) |
F |
25 |
Dental implant placement
Prosthetic rehabilitation (after 3 months) |
Man: 5 |
4.5 |
Gingival hyperplasia around 1 implant Healthy |
Lack of keratinized gingiva |

1. Patients’ sex and age at the time of implant placement
2. Treatment modality, additional procedures, and grafting donor site or material
3. The number of inserted implants and the outcome of treatment (for example, implant failure, peri-implantitis, or healthy implant)
4. The probable reason for peri-implant diseases
5. The follow-up period after implant placement
6. General characteristics of the selected studies
4. Quality assessment of studies
The quality of each study was assessed using CARE (CAse REport) guidelines
13 to estimate risk of bias. The quality of all thirteen parts was considered.
5. Statistical method
Data analysis was performed using Stata statistical software (release 15; StataCorp, College Station, TX, USA). Descriptive data were expressed as percentages. In order to examine the statistical heterogeneity of the data, the I2 statistic was used. To analyze and integrate the results, random and fixed effect methods were used for maxillary and mandibular implants, respectively.
Go to :

III. Results
The primary electronic search resulted in 80 studies. After exclusion of irrelevant studies, 11 articles with 15 cases were included in this systematic review.(
Table 1) These cases included six male and nine female patients afflicted by PLS who were treated by dental implants. The mean age of patients at the time of implant placement was 20.69±4.96 years, and most of implant placement was done after the age 18. A total number of 136 dental implant placements were reported for these patients. Most of the treatment plans called for full mouth rehabilitation followed by mandibular overdenture. One case reported two single implant placements of maxillary central incisors, the only maxillary teeth lost. The patient was under meticulous follow-up before and during permanent teeth eruption. Her mandibular incisors were planned for future reconstruction
3.
Peri-implantitis was reported in three patients with poor oral hygiene, and a total of 20 dental implants failed (14.70%). Implant failures occurred in three patients, and these rates were obtained without considering the replaced implants. The reasons of implant failure were peri-implantitis (2 patients) and lack of osseointegration (1 patient). Gingival hyperplasia around one implant due to lack of attached gingiva was reported. The failure rate was higher in maxillae. Meta-analysis of the probability of failure was 7% (95% confidence interval [CI] 0%-31%) for maxillary implants and 2% (95% CI 0%-9%) for mandibular implants. The value of I
2 was 73% and 0% for maxillary and mandibular implants, respectively. The overall result for all I
2 was 50.81% with a pooled random effect estimate of 3% (95% CI 0%-12%). A forest plot of the meta-analysis is provided in
Fig. 2. None of the patients had healing problems after the placement of implants or bone graft. The mean and median follow-up after prosthetic rehabilitation was 5.16±5.08 years and 4 years (Q1=1, Q2=8.75), respectively. The follow-up time ranged between 1 years and 20 years.
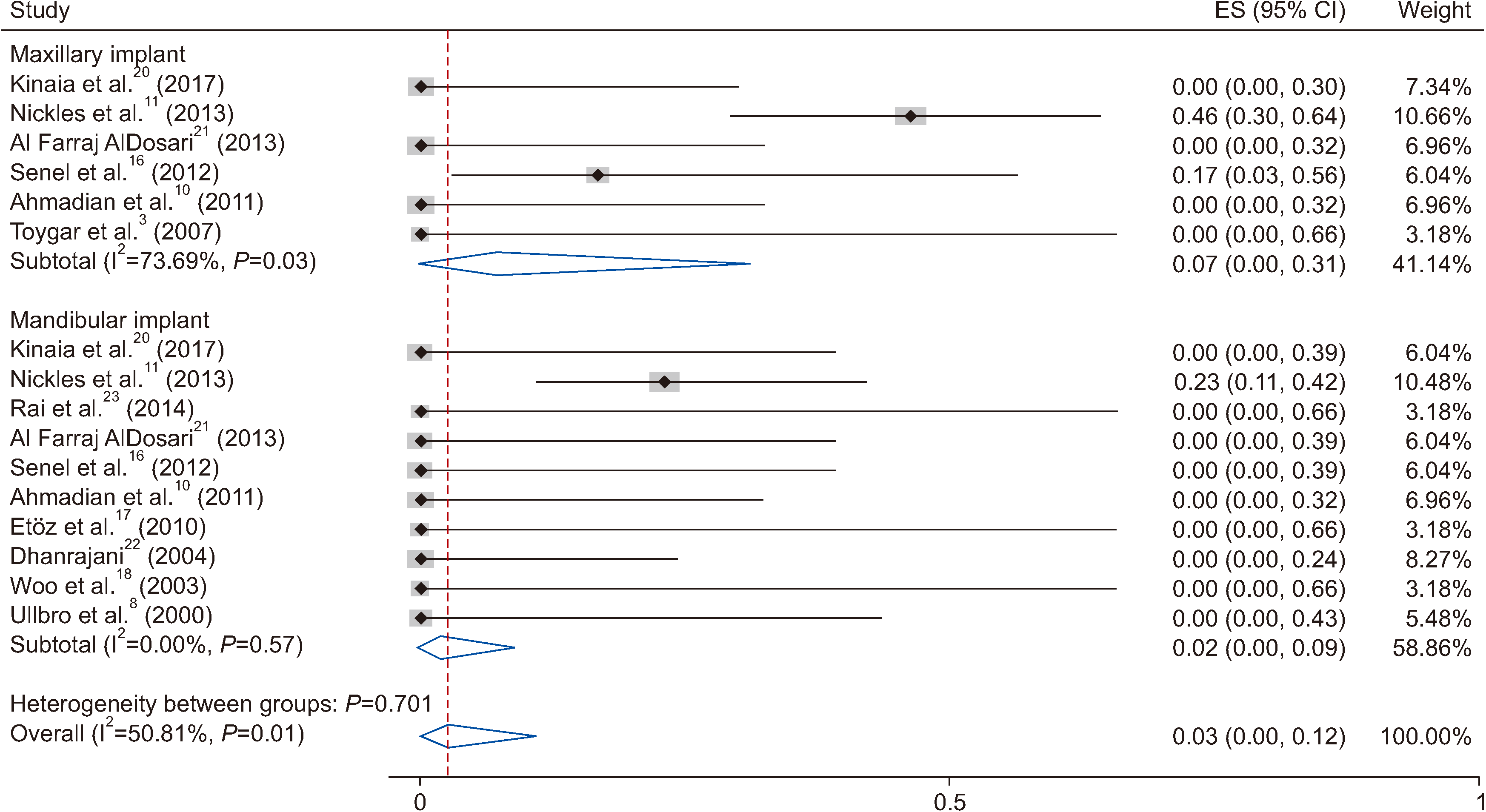 | Fig. 2Probability of implant failure based on maxillary and mandibular implant. (ES: effect size, CI: confidence interval) 
|
Go to :

IV. Discussion
Periodontitis is an important manifestation of PLS and, in these patients, the conventional periodontal treatments usually cannot prevent the progressive attachment loss
8,14
. In addition to periodontal treatment, antimicrobial therapies including erythromycin, tetracycline, penicillin, and amoxicillin-metronidazole have been suggested. However, controversial results have been reported
15
. Continuous attachment and tooth loss are present in some patients
11,14
. These patients experience partial or complete edentulism during their adolescence
8
and need oral reconstruction. Conventional complete denture or overdenture is the traditional prosthetic treatment for PLS patients, but this treatment causes esthetic and functional problems that lead patients to seek a more comprehensive treatment
10
. The chief patient complaints were the lack of stability and retention of their mandibular prosthesis
8,
16-
18. Dental implants can provide the necessary support, stability, and retention for dental prostheses
10
and are, therefore, helpful to PLS patients. The benefits of implants include not only enhanced prosthesis stability and retention, but also preservation of the supporting bone and prevention of further bone loss
8
.
Extraction of all PLS patient primary teeth so that the patient experiences an edentulism period between primary and permanent dentition has been reported to be helpful
9,14,19
.
Although implants help patients with edentulism, lack of available bone for dental implant placement as the result of progressive periodontitis and/or continuous use of full dentures for many years is the major problem for these patients
10,17,18
.Therefore, an implant-based treatment plan for these patients is, in many cases, restricted to overdentures unless complicated pre-surgical bone augmentations are required
10
. In this review, pre-surgical augmentation was reported in three patients. These included sinus augmentation, inferior alveolar nerve repositioning and guided bone regeneration (GBR) using extra-oral harvesting bone (calvaria and tibia) or bone substitute material
3,10,20
. Simultaneous GBR was also performed during implant placement if needed
21
. In all cases with bone augmentation, the healing period was normal and uneventful
3,10,20,21
.
To avoid these complicated and expensive procedures, implant placement between the mental foramina for fixed mandibular prosthesis could be the treatment of choice if sufficient bone is available in the anterior mandible
8,22
. Insertion of two implants in the anterior mandible was another treatment modality for an implant-supported removable overdenture
17,18,23
. Etöz et al.
17
used short dental implants for mandibular overdenture support. Their patient showed severe ridge atrophy so distraction osteogenesis had a potential for bone fracture
17
.
Osseointegration occurred successfully except for the early failure of one implant. For areas lacking soft and hard tissue, distraction osteogenesis is suggested
3
.
Another consideration for PLS patients is the age at edentulism. Most of the patients lost their teeth early; these patients lost most of their permanent teeth by 14 years
5
. However, dental implants act as ankylosed teeth and are contraindicated in teenagers and growing individuals
24
. Dental implants inserted in patients under the age of 18 led to infra-occlusal positioning of the maxillary dental implant. Insertion of dental implants in the anterior mandible encountered less complications
1
. Ullbro et al.
8
suggested that dental implant complications in growing PLS patients was less important than bone preservation. Bohner et al.
1
suggested that, whenever growing patients may benefit more from dental implants, the implantation can be performed cautiously; maintenance follow-ups and implant-supported prosthesis adjustments are required until growth cessation. However, in their systematic review only anterior region implant placements were considered
1
. Dental implant placement before the cessation of growth had been performed in patients with ectodermal dysplasia. A systematic review
25
demonstrated that the rate of dental implants failure in these patients was relatively low (5.3%-7.2%).
Impaired immune systems of young PLS patients is another consideration
11
. PLS patient neutrophils are deficient in the ability to establish neutrophil extracellular traps (NET), and chemotactic velocity is also reduced in PLS patient neutrophils
7
. However, clinical evaluation and long-term follow-up of PLS patients have shown that the function of PMNs in PLS patients improves with age
8,9
. Tinanoff et al.
9
reported normal PMN chemotaxis and adherence in their patient after 15 years follow-up (at age 24 years). Ullbro et al.
8
tested the PMN chemotaxis and phagocytosis of their patient before implant placement (at age 25 years) which had improved to normal values. Therefore, the insertion of PLS patient dental implants at younger ages may result in the same inflammatory process as the one that occurs in the teeth. Based on these articles, the optimum age for implant placement in PLS patients is still unclear; and, if implant treatment is performed at an early age, immunological analysis is necessary. Most of the cases in this review received dental implant treatment after the age of 18.
This systematic review assessed the results of dental implant treatment in PLS patients. The longest follow-up periods after implant placement reported in these studies were 20 years and 10 years
11
. Other extended follow-up periods included 9 years
22
, 4.5 years
8
, and 4 years
10,11
. In 40% of patients the implant follow-up time was 2 years or less.
Peri-implantitis occurred in three patients. Implant failure (19 implants) resulted in two of these patients. Poor oral hygiene and poor compliance with the maintenance program were reported as a probable cause of implant failure. Another implant failure occurred due to lack of osseointegration; implant replacement was successful. One implant showed gingival hyperplasia due to lack of attached gingiva. Poor oral hygiene and lack of regular attendance at recall visits were reported as important factors in occurrence of peri-implant diseases
26-
28. These results emphasize that oral hygiene and compliance with follow-up programs have important roles in PLS patient implant success. The results of this study showed a higher rate of maxillary peri-implantitis and implant failure.(
Table 1,
Fig. 2) The data concerning the higher prevalence of maxillary peri-implantitis was heterogeneous and this relationship was not proven
29
.
More cases with long-term follow-up results are required for drawing definite conclusions about dental implant treatment modalities in PLS patients.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download