This article has been
cited by other articles in ScienceCentral.
Abstract
We present two cases of hepatic atrophy treatment with portal vein embolization (PVE) to control intractable cholangitis. The first case was a 60-year-old male who was admitted for repeated episodes of cholangitis. He had undergone cholecystectomy and Roux-en-Y choledochojejunostomy 2 years earlier. Imaging studies showed left intrahepatic duct dilatation and anastomotic site stricture. The patient was reluctant to undergo another surgery. Thus, we decided to perform left PVE to induce atrophy of the left liver. The left liver shrank and stayed silent for 5 years, but a radiological intervention was necessary to treat symptomatic anastomotic stenosis. The patient has done well for 12 years after PVE. The second case was a 51-year-old female who was also admitted for repeated episodes of cholangitis. She had undergone excision of type I choledochal cyst 2 years earlier. Imaging studies showed right hepatic duct stenosis. Cholangitis developed repeatedly. Thus, radiologic interventions were performed 8 times over 9 years. Finally, she was referred for surgery, but she was very reluctant to undergo another surgery. We planned a wait-and-see strategy following right PVE. After PVE, the right liver progressively shrank. Three months after PVE, we decided to wait for a longer period until further atrophy of the right liver. The patient has been doing well for 14 months after PVE without any episode of cholangitis. In conclusion, experience from our two cases suggests that hepatic parenchymal induction therapy through percutaneous PVE can be a therapeutic option for patients with perihilar biliary stenosis-associated cholangitis.
Go to :

Keywords: Biliary stenosis, cholangitis, liver atrophy, Portal vein embolization, radiological intervention
INTRODUCTION
Benign perihilar or intrahepatic biliary strictures inducing repeated episodes of cholangitis are difficult to treat through non-surgical methods. Thus, surgical treatment including resection of the involved liver portion is usually performed as the final definitive treatment after numerous repeated sessions of radiological intervention. The main mechanisms of cholangitis in patients with biliary stricture is stenosis-associated bile stasis, with or without combined ascending infection from patulous sphincter of Oddi or Roux-en-Y biliary-enteric anastomosis. If production of bile decreases with no change in biliary stenosis, bottlenecking bile stasis will be improved. Based on this theoretical basis, we have used a wait-and-see strategy after portal vein embolization (PVE) to control intrahepatic duct stenosis- associated cholangitis.
1-
7 We herein present two cases of hepatic atrophy treatment with PVE to control intractable cholangitis.
Go to :

CASE
Case 1
The patient was a 60-year-old male who was admitted for repeated episodes of cholangitis. He had undergone cholecystectomy and Roux-en-Y choledochojejunostomy 2 years earlier at an outside hospital probably because of iatrogenic bile duct injury during surgery for gallstone disease. Computed tomography (CT) showed left intrahepatic duct dilatation with abrupt luminal narrowing around the surgical anastomosis site and a suspicious high attenuating lesion in the left proximal intrahepatic duct, suggesting anastomotic site stricture (
Fig. 1A). Hepatobiliary scintigraphy showed delayed biliary excretion of the left liver with normal excretion of the right liver (
Fig. 1B). The patient was reluctant to undergo another surgery. Thus, we decided to perform left PVE to induce atrophy of the left liver (
Fig. 2). We followed the patient with liver CTs every 3 months during the first year and yearly after that, in which the left liver was shrunken markedly without any episode of cholangitis (
Fig. 3). However, after 5 years, cholangitis suddenly developed and CT scan showed development of multiple abscesses at the left lateral segment and caudate lobe. Thus, percutaneous transhepatic biliary drainage (PTBD) was performed through the left hepatic duct, and cholangitis was rapidly controlled (
Fig. 4). After 6 years, CT scan showed cholangiohepatitis in the right liver and a 1 cm-sized stone at the choledochojejunostomy site. Right PTBD was performed and repeated balloon dilatation was performed to the stenotic ducts and choledochojejunostomy site (
Fig. 5). The PTBD tube was kept for 45 days. Thereafter, no episode of cholangitis developed. The patient has done well for 12 years after PVE.
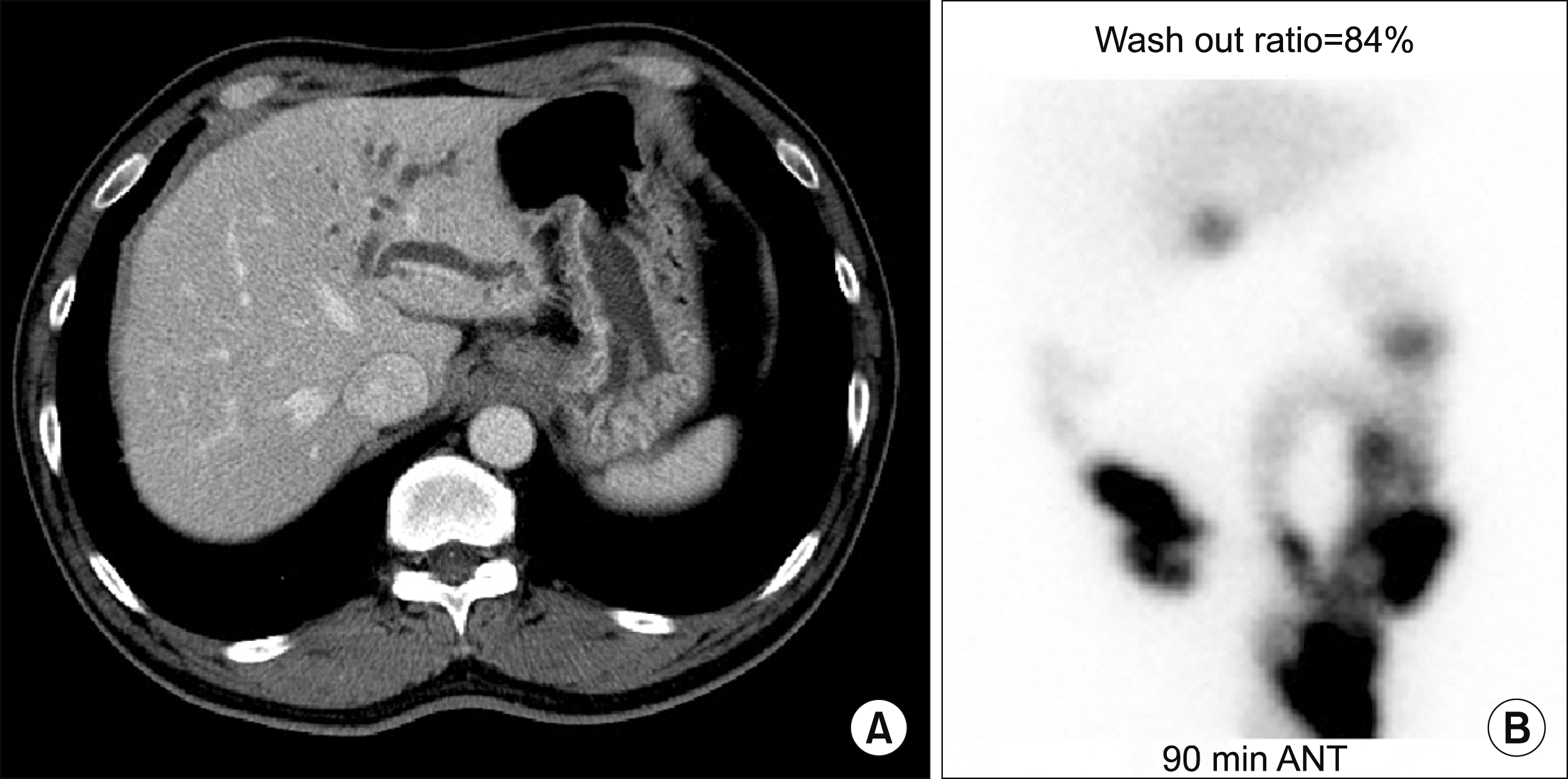 | Fig. 1In image findings of the Case 1. (A) Computed tomography showed left intrahepatic duct dilatation with abrupt luminal narrowing around the surgical anastomosis site and suspicious high attenuating lesion in the left proximal intrahepatic duct. (B) Hepatobiliary scintigraphy showed delayed biliary excretion of the left liver. 
|
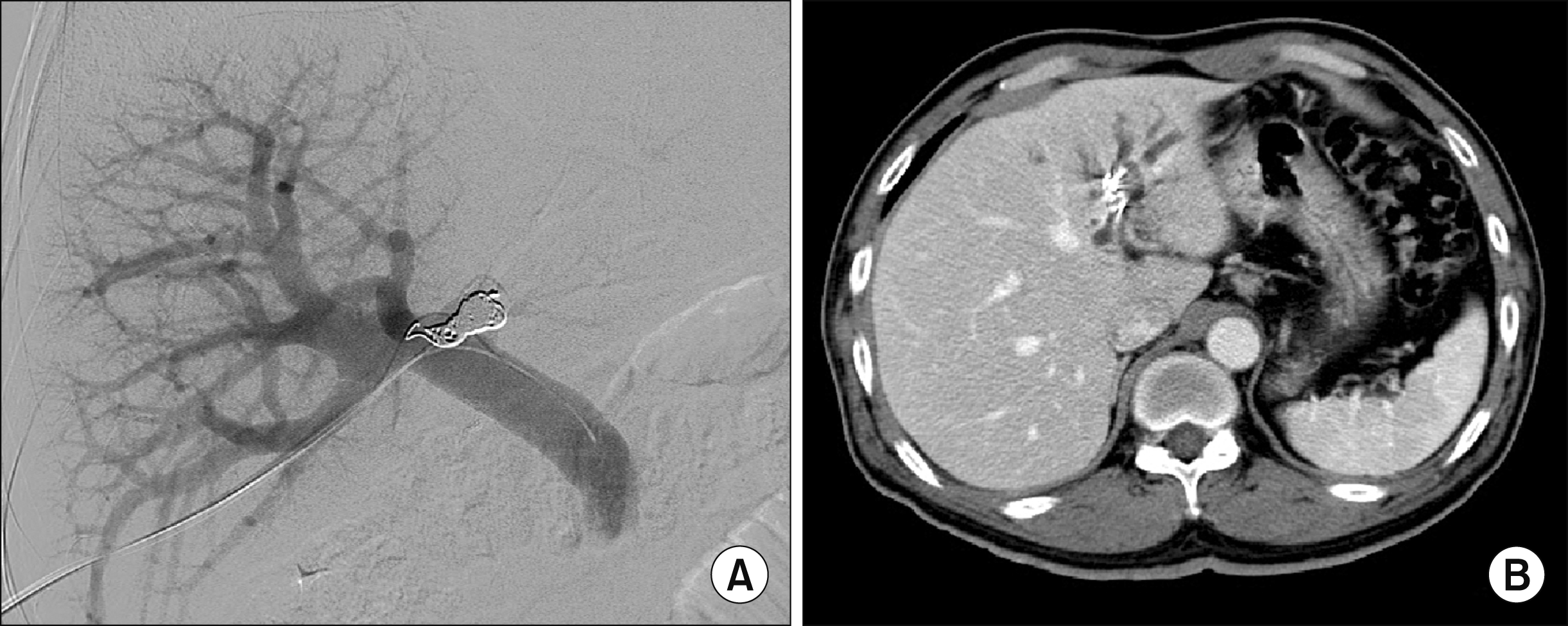 | Fig. 2Left portal vein embolization of the Case 1 (A) and computed tomography taken after 5 days (B). 
|
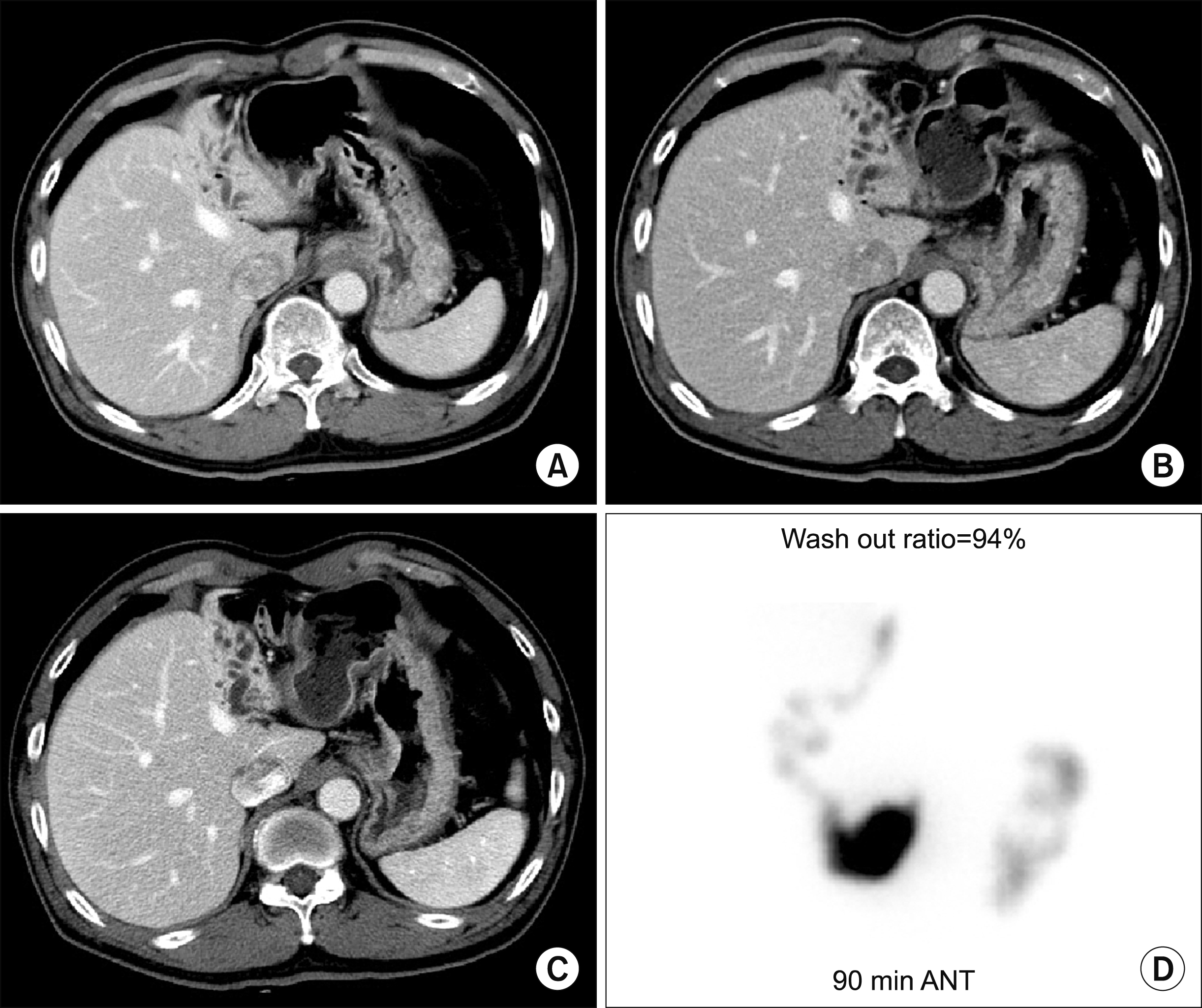 | Fig. 3Follow-up computed tomography scan of the Case 1 at 7 days (A), 2 years (B) and 4 years (C) after portal vein embolization. Hepatobiliary scintigraphy taken at 4 years showed normal biliary excretion (D). 
|
 | Fig. 4Follow-up imaging studies of the Case 1 at 5 years after portal vein embolization. (A) The left hepatic ducts were dilated. (B) Percutaneous transhepatic biliary drainage was performed. (C) The left hepatic ducts were decompressed. (D) The left liver was shrunken. 
|
 | Fig. 5Follow-up imaging studies of the Case 1 at 6 years after portal vein embolization. (A) There was cholangiohepatitis in the right liver and a 1 cm-sized stone at the choledochojejunostomy site. (B-D) Percutaneous transhepatic biliary drainage and repeated balloon dilatation were performed. (E) Cholangitis was controlled. (F) Hepatobiliary scintigraphy showed normal biliary excretion. 
|
Case 2
The patient was a 51-year-old female who was admitted for repeated episodes of cholangitis. She had undergone excision of type I choledochal cyst and Roux-en-Y hepaticojejunostomy 2 years earlier at an outside hospital (
Fig. 6). CT scan and magnetic resonance cholangiography showed right intrahepatic duct stone with biliary dilatation (
Fig. 7A, B). PTBD was performed and the stenotic sites were dilated. Intrahepatic stones were removed through percutaneous transhepatic cholangioscopy (
Fig. 7C, D). PTBD and balloon dilatation were repeated 8 times over 9 years. Finally, she was referred for surgery, but she was very reluctant to undergo another surgery. We explained the wait-and-see strategy after PVE and right PVE was performed (
Fig. 8). After PVE, the right liver progressively shrank (
Fig. 9). Three months after PVE, we decided to wait for a longer period until further atrophy of the right liver. Twelve months after PVE, we decided to perform regular follow-ups with imaging studies unless cholangitis developed again. The patient has done well for 14 months after PVE without any episode of cholangitis.
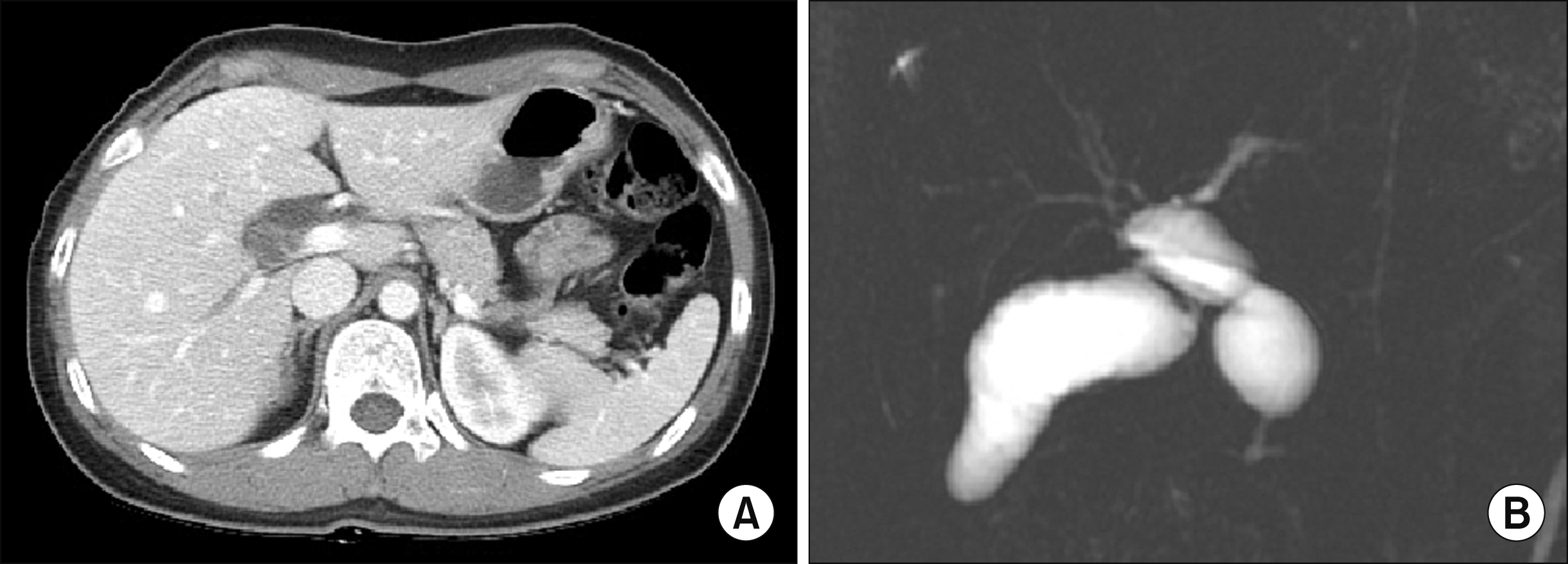 | Fig. 6Preoperative imaging studies of the Case 2 showing choledochal cyst of type I (A) and no marked dilatation of the intrahepatic ducts (B). 
|
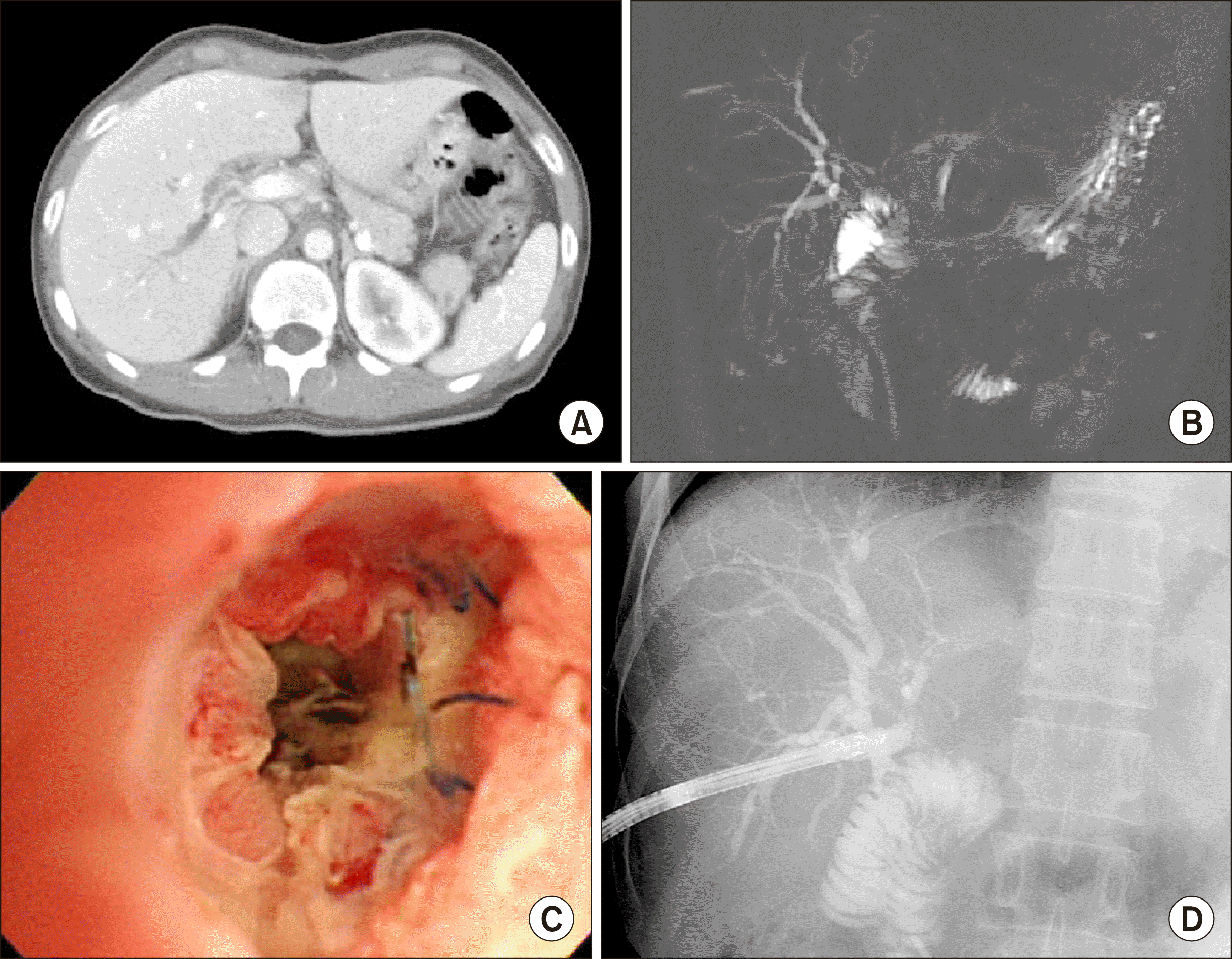 | Fig. 7Initial image findings of the Case 2. (A and B) Computed tomography and magnetic resonance cholangiography showed right intrahepatic duct stones with biliary dilatation. (C and D) Intrahepatic stones were removed through percutaneous transhepatic cholangioscopy. 
|
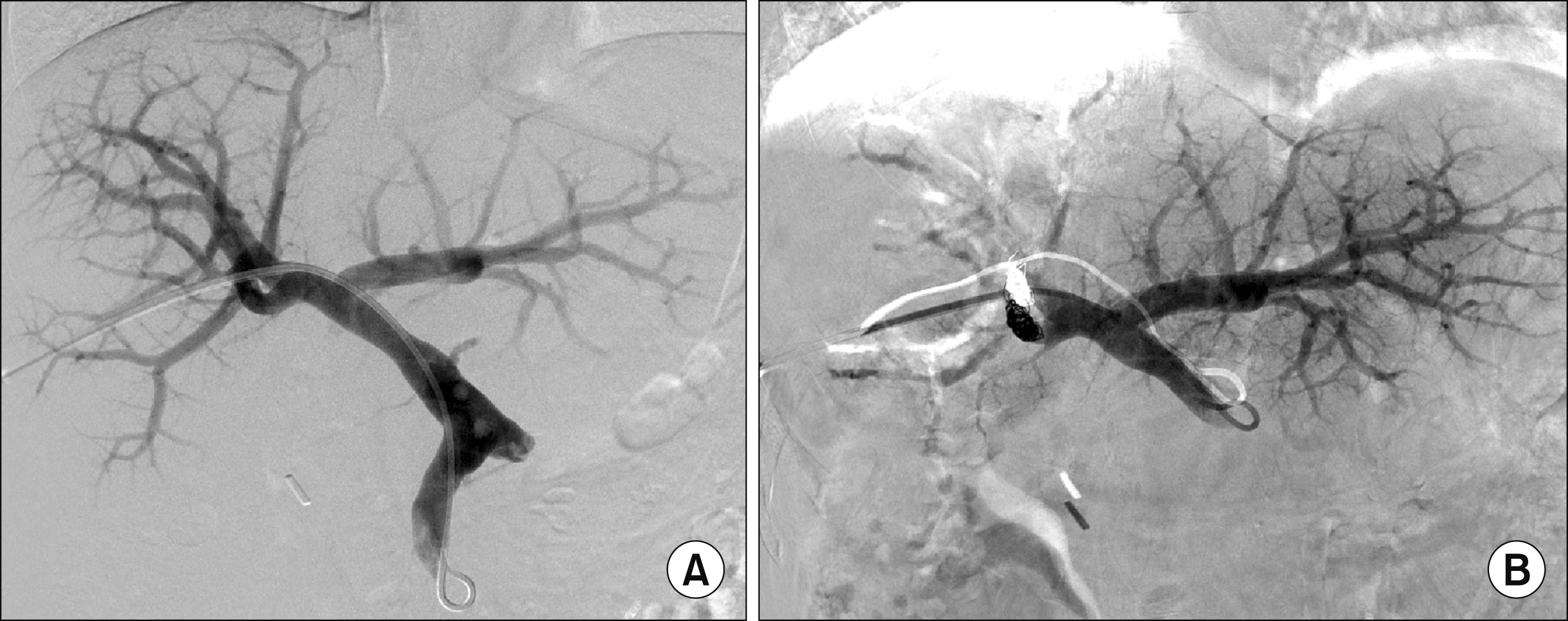 | Fig. 8Right portal vein embolization of the Case 2 before (A) and after (B) embolization. 
|
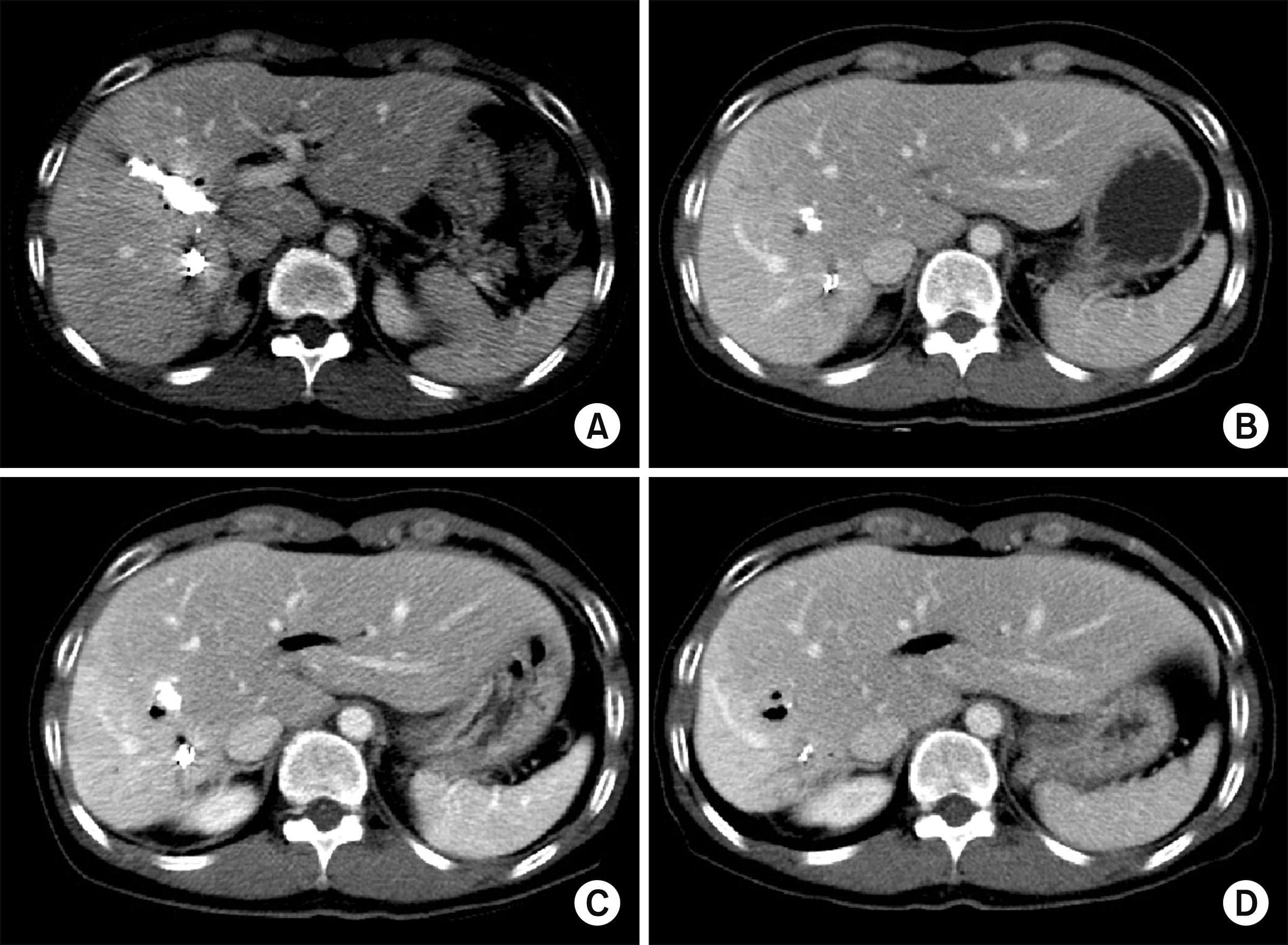 | Fig. 9Follow-up computed tomography (CT) scans of the Case 2 during the first 1 year after portal vein embolization showing progressive atrophy of the right liver. CT scans were taken at 7 days (A), 1 month (B), 3 months (C) and 12 months (D) after portal vein embolization. 
|
Go to :

DISCUSSION
Deprivation of portal blood flow induces atrophy of the ipsilateral liver.
8,
9 We previously reported the effectiveness and usefulness of hepatic atrophy induction treatment for isolated segmental bile duct injury following laparoscopic cholecystectomy and central hepatectomy.
1-
3
In our previous cases,
1-
3 bile leak was present. Thus, the management process was complex and close observation was necessary. In patients with bile duct injury, two mechanisms are associated with hepatic parenchymal atrophy, deprivation of portal blood flow and bile duct obstruction. The treatment procedure consisted of three major steps. The first step was embolization of the segmental portal branch, which effectively inhibited the quantity and quality of bile production.
7,
10 The second step was the closure of the leakage site through the induction of heavy adhesion to ensure clamping of the PTBD or pigtail drainage. The third step was the inhibition of bile drainage through spontaneous occlusion of the segmental duct to accelerate atrophy of the segmental parenchyma. These three-step procedures usually took 3-4 months.
1
Unlike the abovementioned cases, our present patients did not have bile leaks, so only wait-and-see approach was necessary following PVE. The speed of hepatic parenchymal atrophy varies depending on the status of the liver and embolic methods and materials for PVE. Therefore, the concepts of kinetic growth and shrinkage rates are used.
5,
9,
10 These rates are high in the order of normal, chronic hepatitis and cirrhotic livers. The left liver in our first case was compatible with a chronic hepatitis liver because there were significant precedent injuries inducing fibrosis. In contrast, the second case liver was compatible with a normal liver because the right liver parenchyma was well-preserved despite repeated episodes of cholangitis. If the right liver did not shrink significantly 1-2 months after right PVE, our choice would have been upfront surgery or further waiting after additional right hepatic vein embolization.
Intrahepatic stone formation is an indication for surgery instead of atrophy induction unless the stones are removed completely. Such formation of intrahepatic stones indicates the presence of severe stenosis and repeated episodes of inflammation, which can be risk factors for malignant transformation. Our two patients also had risks for late malignant transformation at the intrahepatic bile ducts of the shrunken liver, thus we suggest that life-long surveillance is necessary for such patients with hepatolithiasis or parenchymal atrophy.
11
In conclusion, the experience from our two cases and other precedent cases suggests that hepatic parenchymal induction therapy through percutaneous PVE can be a therapeutic option for patients with perihilar biliary stenosis-associated cholangitis.
Go to :











 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download