Abstract
Backgrounds/Aims
Pancreaticoduodenectomy is the only potentially curative treatment for pancreatic cancer. The identification of the first nodal drainage site (sentinel node) may improve the detection of metastatic nodes and can contribute to a less invasive surgery. We aimed to determine the accuracy of sentinel node mapping in patients with pancreatic cancer using intraoperative radiotracer injection technique.
Methods
At surgical exposure, peritumoral injection of 0.4-0.5 mci/0.5 ml of 99mTc- sodium phytate was performed. After tumor resection, sentinel nodes were investigated in the most common areas using a hand-held gamma probe. Any lymph node with in vivo count twice the background was considered as sentinel node, thus, it was removed and sent for pathological assessment. Then a standard lymph node dissection was performed for all patients.
Results
Fourteen patients with cancer in the head of the pancreas were included in this study. Overall, 180 lymph nodes were harvested with a mean of 11.6±4.7 lymph nodes per patient. In eight patients, at least one sentinel node could be identified (detection rate about 64%). False negative rate of the study was 3/5 (60%).
Go to : 
Surgical resection is the only potentially curative treatment for pancreatic cancers;1 however, only 15-20% of patients with this type of cancer are finally candidate for pancreaticoduodenectomy (PD; standard or extended) at the time of diagnosis.2,3
Generally, the prognosis of patients with pancreatic cancer is dependent on the histological status and growth rate of the tumor.4 Based on histopathological assessment, lymphatic invasion is observed in the majority of patients undergone surgery for pancreatic neoplasms.5 PD with extensive lymphadenectomy can improve the prognosis of pancreatic cancer; however, it can lead to increased morbidity due to extensive surgeries.6
Staging of lymph nodes is very important to decide on the viable treatment option for pancreatic cancer. In this regard, identifying cases without lymph node involvement can be useful in preventing morbidity due to unnecessary dissection.7 Sentinel lymph node (SLN) is the first lymph node invaded by malignant cells. Identification of the first nodal drainage site (sentinel node) may improve the detection of metastatic nodes. Accordingly, determining the sentinel node is of great significance to decide on the type and extent of surgery.8
Lymphoscintigraphy with SLN analysis is a standard technique in malignant melanoma, breast cancer, and head and neck cancer.9,10 This technique has been recently applied for the detection of tumors of the gastrointestinal tract including the pancreas to prevent unnecessary extended lymphadenectomy.11-14 However, limited studies have performed more than the injection of blue dye into the tumor and peritumoral area, which have yielded discrepant results.7,12,15,16
This study aimed to determine the effectiveness of a radiotracer by using lymphoscintigraphy and intraoperative gamma probe in SLN detection in pancreatic cancer patients. Therefore, we evaluated the accuracy of sentinel node mapping in 14 patients with pancreatic cancer using intraoperative radiotracer injection technique.
Go to : 
This study was performed on 14 patients with cancers of the head of the pancreas who were candidates for pancreatectomy and hospitalized in Department of Surgery at Imam Reza Hospital, Mashhad, Iran, during 2015-17. Diagnoses were made based on clinical manifestations, imaging view, and assessment of tumor markers. Endoscopic ultrasound, magnetic resonance imaging (MRI), and CT angiography were performed as clinically indicated on the 14 patients preoperatively. Patients with distance metastasis, localized invasion, neoadjuvant therapy, and sensitivity to radioisotope were excluded.
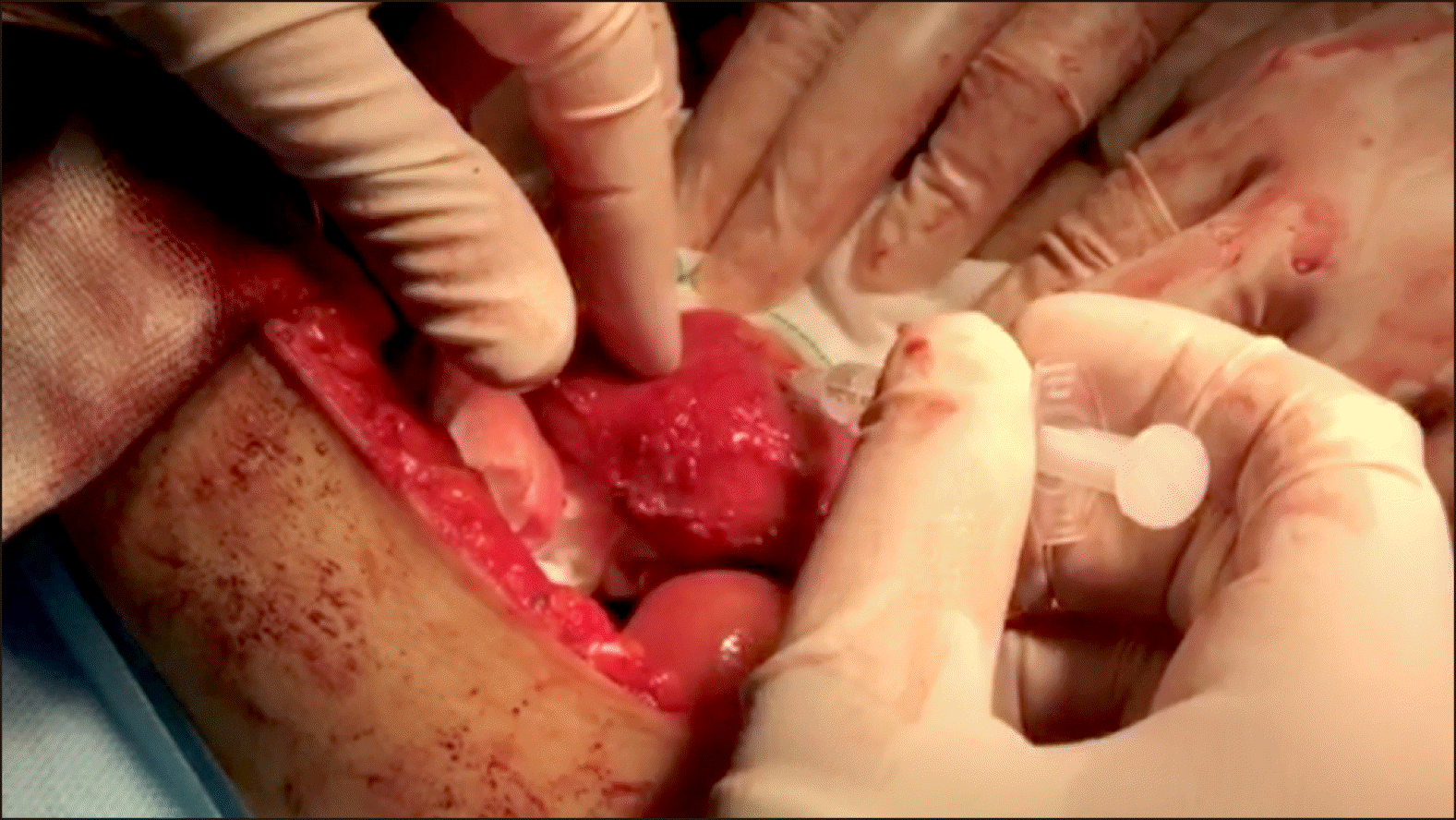
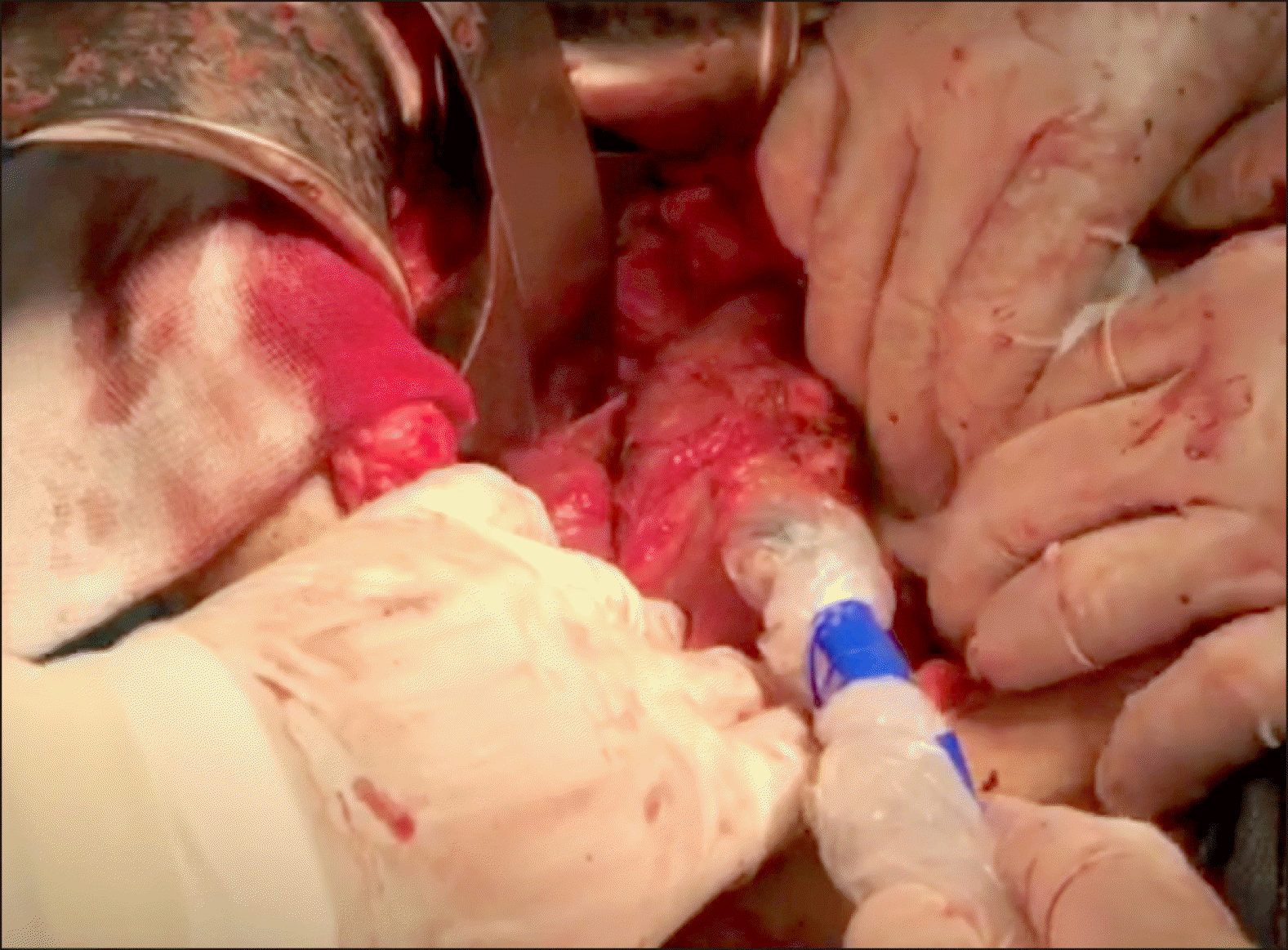
Radio pharmaceutical dosage was prepared based on a predefined protocol and injected after laparotomy in operable patients. A technician and resident in Nuclear Medicine prepared the sodium phytate kit. After laparotomy, a complete analysis was performed in terms of metastasis and local invasion to the vessels. In case of resectable tumors, peritumoral injection of 0.4-0.5 millicurie 99mTc-sodium phytate in a volume of 2.0 cc (in 4 divided doses) was performed by the surgeon on all four sides of the tumor in sterile conditions of operating room (Fig. 1). After 15 to 20 minutes, a gamma probe device (Surgioguide II, Parto Negar Persia Co. Ltd.) determined tumor and lymph node counts (Fig. 2).
After resection of the tumor, we searched for sentinel nodes in the most common areas using a hand-held gamma probe. Tumor count of visible lymph nodes was separately assessed by the surgeon. The lymph nodes with twice or more count of the background were considered as sentinel and those with less count were considered as non-sentinel. The resected sentinel and non-sentinel nodes were sent to another pathologist for the necessary assessments. Then, pylorus preserving whipple procedure and standard lymphadenectomy were carried out. The pathology results of sentinel nodes and other lymph nodes were compared with one another.
After pathological examination, data were gathered and recorded in a checklist and classified. The false negative rate and detection rate of sentinel nodes were estimated. Data were analyzed by SPSS, version 21.
The present study was approved by the Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (reference number: 1394.145). All the ethical considerations, including confidentiality of the data, were observed. The stages and techniques of this study were clearly explained to the patients, and their informed consent was obtained.
Go to : 
Cancer in the head of the pancreas was confirmed in all the patients. The mean age of the patients was 58.7±9.8 years (age range: 40-75 years). Half of the participants were male and half of them were female. Totally, 87.5% of our patients were classified as T3 and 21.5% of them as T2 based on TNM classification of malignant tumors. The mean tumor size was 3.3±1.98 cm.
Adenocarcinoma was observed in 78.5% (n=11) of the subjects based on pathological examination. Also, one case (7.1%) with adenosquamous carcinoma, one case with papillary mucinous neoplasm along with invasive ductal carcinoma (IDC), and one case with solid pseudopapillary tumor (SPT) carcinoma were diagnosed.
About 180 lymph nodes were removed in 14 surgeries (11.6±4.7 in each surgery). Among them, 17 (9.4%) lymph nodes were SLNs and 163 (90.5%) were non-SLNs.
At least one sentinel node was diagnosed in 64.3% of the patients, while it was not diagnosed in 35.7% of the cases; therefore, detection rate was calculated at 64%. Characteristics of the patients with pancreatic cancer, tumor location, and other obtained results from the examination of lymph nodes are presented in Table 1.
Characteristics of the patients with pancreatic cancer, tumor location, and other obtained results from the examination of lymph nodes
Based on final pathology reports, lymph node involvement with malignancy was reported in 42.8% of the cases (6 patients), and positive SLN was observed in only 14.2% of them (two cases). In one case, no sentinel node was detected. Therefore, the rate of false negative results in patients receiving radio pharmaceutical study was estimated at 60% (3/5).
The final pathology reports of resected non-SLNs were positive in six patients, while other eight cases had negative lymph nodes. Also, the final pathology reports of resected SLNs with 99mTc-sodium phytate was positive and negative in two and seven lymph nodes, respectively. There were no cases of morbidity or mortality due to the injection of radiolabeled nanocolloid.
Go to : 
Our study revealed that SLN biopsy by using radioactive tracer has a high false negative rate and a relatively low diagnostic accuracy. In our study, SLN detection rate by 99mTc-sodium phytate was estimated at 64% and the false negative rate was appraised at 60%. several previous studies showed that the use of lymphoscintigraphy with the injection of Tc99m and intraoperative gamma probe detection may be a viable and safe approach for locating SLNs in patients with pancreatic cancer.16 However, the interpretation of the findings is challenging.
Beisani et al.16 performed a study to detect SLNs in pancreatic cancer patients using a lymphoscintigraphic technique. In the mentioned study, seven patients with pancreatic head cancer received intratumoral injection of 99mTc- labelled nanocolloid before surgery, and then they were assessed for sentinel nodes using SPECT/CT and hand- held gamma probe. Sentinel nodes were found in two cases, where SLN was confirmed as truly negative by final histopathological evaluation in one patient. That study showed the applicability of radiotracers for the detection of SLN; however, because of technical problems, the interpretation of the imaging findings was insufficient for clinical validation. 16
The surgical SLN detection technique for pancreatic cancer was applied in other similar studies; however, some only used the blue dye technique.7,12 The findings showed that intraoperative gamma probe detection and blue dye mapping are effective in providing a pattern of lymph node metastasis; nonetheless, this procedure is costly and is not sufficiently accurate.17
The detection rate was estimated at 95% in a study by Tsioulias et al.18 According to the mentioned study, SLNs were found in the majority of patients with pancreatic cancer. Additionally, among patients with nodal metastasis, positive SLN was observed in 89% and lymph node involvement was identified in 42% of patients. According to this study, lymphatic mapping can be useful in the detection of lymph node involvement and pancreatic cancer staging.18
Based on study by Durczyński et al.15 sentinel nodes were observed in 38.46% of patients with locally advanced pancreatic tumors, but all the identified sentinel nodes were metastatic. The obtained results of that study indicated the incompetence of sentinel node analysis to determine the pathway of lymphatic pancreatic cancer. They found that sentinel node navigation is highly restricted to the recognition of accurate pattern of lymph flow from primary pancreatic tumors due to delayed detection of these tumors.15 Delayed detection of pancreatic tumors is due to the fact that they are asymptomatic in the early stages; therefore, these tumors are usually diagnosed in a late stage of local advancement, when the obstruction and invasion of lymphatic channels are possible. In advanced pancreatic body tumors, the feasibility of sentinel node navigation is considerably restricted. The identification of sentinel nodes may be hindered by lymph flow diversion due to extensive lymph node metastases.
Although the mapping technique has been used to identify sentinel nodes in intraabdominal malignancies such as colorectal cancer,19 or in breast cancer with a low false negative rate,20 it does not seem to be applicable for pancreatic cancer, which could be accounted for by multiple factors. The majority of pancreatic cancer cases are diagnosed in advanced stages compared to other cancers (such as breast cancer). The presence of several pathways of lymphatic drainage in the pancreas may also be another cause of failure in the application of this technique. Further, the presence of veins draining into the gastrointestinal tract, and superior mesenteric vein can make it difficult to find sentinel nodes. In addition, intestinal activity and high vascularity may interfere with the detection of true SLNs and it can cause high background noise during surgery when gamma probe is being applied. Despite a high association between low drainage and intratumoral injection compared to peritumoral injection, no definitive SLN pathway has yet been discovered16 for pancreatic head cancer. On the other hand, lymphatic flow may be blocked by intense desmoplastic reaction.
We suggest further studies on lymphatic mapping in patients in early stages of pancreatic cancer. Furthermore, we propose future studies to use larger sample sizes and frozen section for more accurate SLN assessment and perform radiotracer injection one day before surgery and SPECT/CT as an auxiliary localization method.
Small sample size was the main limitation of this study; nevertheless, our study had a larger sample size compared to similar studies.
Due to insufficient diagnostic accuracy and high false negative rate, this study didn’t support the use of sentinel node mapping technique, as an alternative for standard lymphadenectomy in patients with pancreatic cancer. However, further studies are required to obtain more accurate results.
Go to : 
ACKNOWLEDGEMENTS
The present study was supported by the Research Deputy of Mashhad University of Medical Sciences, Nuclear Medicine Research Center, and Cancer Surgery Research Center of Imam Reza Hospital, Mashhad, Iran.
Go to : 
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: RS, MA. Data curation: MA, HS. Formal analysis: RS. Funding acquisition: MA. Methodology: RS. Project administration: MA, HS, BM, VRD. Visualization: MA. Writing - original draft: HS. Writing - review & editing: RS, MA.
Go to : 
REFERENCES
1. Shrikhande SV, Barreto SG. 2010; Extended pancreatic resections and lymphadenectomy: an appraisal of the current evidence. World J Gastrointest Surg. 2:39–46. DOI: 10.4240/wjgs.v2.i2.39. PMID: 21160848. PMCID: PMC2999214.

2. Ma J, Siegel R, Jemal A. 2013; Pancreatic cancer death rates by race among US men and women, 1970-2009. J Natl Cancer Inst. 105:1694–1700. DOI: 10.1093/jnci/djt292. PMID: 24203988.

3. Zhang J, Dhakal I, Ning B, Kesteloot H. 2008; Patterns and trends of pancreatic cancer mortality rates in Arkansas, 1969-2002: a comparison with the US population. Eur J Cancer Prev. 17:18–27. DOI: 10.1097/CEJ.0b013e32809b4ccd. PMID: 18090906.

4. American Joint Committee on Cancer. 2010. AJCC cancer staging handbook. 7th ed. Springer;New York:
5. Ishikawa O, Ohigashi H, Sasaki Y, Kabuto T, Furukawa H, Nakamori S, et al. 1997; Practical grouping of positive lymph nodes in pancreatic head cancer treated by an extended pancreatectomy. Surgery. 121:244–249. DOI: 10.1016/S0039-6060(97)90352-4. PMID: 9068665.

6. Millikan KW, Deziel DJ, Silverstein JC, Kanjo TM, Christein JD, Doolas A, et al. 1999; Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg. 65:618–623. discussion 623–624. DOI: 10.1016/S0090-4295(99)00304-0. PMID: 10399969.
7. Ohta T, Kitagawa H, Kayahara M, Kinami S, Ninomiya I, Fushida S, et al. 2003; Sentinel lymph node navigation surgery for pancreatic head cancers. Oncol Rep. 10:315–319. DOI: 10.3892/or.10.2.315. PMID: 12579265.

8. Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, Shimizu A, et al. 2012; Identification of the lymphatic drainage pathways from the pancreatic head guided by indocyanine green fluorescence imaging during pancreaticoduodenectomy. Dig Surg. 29:132–139. DOI: 10.1159/000337306. PMID: 22538463.

9. Pavlidis TE, Pavlidis ET, Sakantamis AK. 2011; Current opinion on lymphadenectomy in pancreatic cancer surgery. Hepatobiliary Pancreat Dis Int. 10:21–25. DOI: 10.1016/S1499-3872(11)60002-7. PMID: 21269930.

10. Yasuda S, Shimada H, Chino O, Tanaka H, Kenmochi T, Takechi M, et al. 2003; Sentinel lymph node detection with Tc-99m tin colloids in patients with esophagogastric cancer. Jpn J Clin Oncol. 33:68–72. DOI: 10.1093/jjco/hyg020. PMID: 12629056.

11. Murawa D, Filas V, Breborowicz J, Spychała A, Dworzecka K, Murawa P. 2007; Evaluation of the sentinel node biopsy in colorectal carcinoma including the results of immunohistochemical examinations. Acta Chir Belg. 107:45–48. DOI: 10.1080/00015458.2007.11680009. PMID: 17405597.

12. Kocher HM, Sohail M, Benjamin IS, Patel AG. 2007; Technical limitations of lymph node mapping in pancreatic cancer. Eur J Surg Oncol. 33:887–891. DOI: 10.1016/j.ejso.2007.02.037. PMID: 17433604.

13. Moncayo VM, Aarsvold JN, Alazraki NP. 2015; Lymphoscintigraphy and sentinel nodes. J Nucl Med. 56:901–907. DOI: 10.2967/jnumed.114.141432. PMID: 25931478.

14. de Bree R, Nieweg OE. 2015; The history of sentinel node biopsy in head and neck cancer: from visualization of lymphatic vessels to sentinel nodes. Oral Oncol. 51:819–823. DOI: 10.1016/j.oraloncology.2015.06.006. PMID: 26126813.

15. Durczyński A, Hogendorf P, Szymański D, Grzelak P, Strzelczyk J. 2012; Sentinel lymph node mapping in tumors of the pancreatic body: preliminary report. Contemp Oncol (Pozn). 16:206–209. DOI: 10.5114/wo.2012.29285. PMID: 23788880. PMCID: PMC3687406.

16. Beisani M, Roca I, Cardenas R, Blanco L, Abu-Suboh M, Dot J, et al. 2016; Initial experience in sentinel lymph node detection in pancreatic cancer. Rev Esp Med Nucl Imagen Mol. 35:287–291. DOI: 10.1016/j.remn.2015.10.006. PMID: 26670326.

17. Park DJ, Lee HJ, Lee HS, Kim WH, Kim HH, Lee KU, et al. 2006; Sentinel node biopsy for cT1 and cT2a gastric cancer. Eur J Surg Oncol. 32:48–54. DOI: 10.1016/j.ejso.2005.09.006. PMID: 16269225.

18. Tsioulias GJ, Wood TF, Morton DL, Bilchik AJ. 2000; Lymphatic mapping and focused analysis of sentinel lymph nodes upstage gastrointestinal neoplasms. Arch Surg. 135:926–932. DOI: 10.1001/archsurg.135.8.926. PMID: 10922254.

19. Chen SL, Iddings DM, Scheri RP, Bilchik AJ. 2006; Lymphatic mapping and sentinel node analysis: current concepts and applications. CA Cancer J Clin. 56:292–309. quiz 316-317. DOI: 10.3322/canjclin.56.5.292. PMID: 17005598.

20. Kapteijn BA, Nieweg OE, Liem I, Mooi WJ, Balm AJ, Muller SH, et al. 1997; Localizing the sentinel node in cutaneous melanoma: gamma probe detection versus blue dye. Ann Surg Oncol. 4:156–160. DOI: 10.1007/BF02303799. PMID: 9084853.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download