This article has been
cited by other articles in ScienceCentral.
Abstract
The transformation of papillary thyroid carcinoma (PTC) to anaplastic thyroid carcinoma (ATC) is well documented in the literature but is an exceptionally rare occurrence in metastatic foci outside the primary thyroid lesion. Even rarer is the simultaneous occurrence of PTC and ATC in the cervical lymph nodes. We report the case of an 85–year–old man who presented with a rapidly growing neck mass diagnosed as PTC. Following surgery, multiple ATC foci in the metastatic cervical lymph node were found coexisting with PTC, whereas in the thyroid, only PTC was found. This case is of high clinical significance because transformation of PTC to ATC outside the thyroid gland per se is very rare and because it suggests rapidly growing tumors in an elderly patient. The use of core needle biopsies in cases with suspected rapid tumor growth can aid in proper diagnosis, surgical decision making, and patient counselling.
Go to :

Keywords: Thyroid cancer, Papillary thyroid carcinoma, Lymphatic metastasis, Anaplastic transformation
Introduction
Anaplastic thyroid carcinoma (ATC) is rare and accounts for only 5% of all thyroid cancers. However, it is the most aggressive form of thyroid cancer and has a very poor prognosis.
1) Evidence that ATC arises from differentiated thyroid carcinoma is accumulating.
2) However, the mechanism of transformation has yet to be clarified. In almost all cases, ATC arises within the thyroid gland and seldom occurs in extrathyroidal foci.
3-13) Transformation of a metastatic differentiated thyroid carcinoma to ATC in the cervical lymph node is extremely rare.
6,8,9,13) We report a case of ATC in the lateral cervical lymph node coexisting with papillary thyroid carcinoma (PTC) at the time of surgery which was suggestive on the initial core needle biopsy of the lymph node.
Go to :

Case Report
An 85–year–old man presented with a rapidly growing mass in the left neck, which was detected just one week prior. An initial computed tomography (CT) scan of the neck revealed conglomerate or multiple lymphadenopathy, with the largest node measuring up to 4.9 cm and showing necrotic portions and perilesional infiltration at left levels IV, VI, and the anterior mediastinum, suggesting metastatic lymphadenopathy (
Fig. 1A, B). Ultrasonography of the thyroid revealed a highly suspicious nodule (K-TIRADS
14) category 5, L1) of 1.8 cm in size in the left lower thyroid gland with hypoechoic, solid, irregular, parallel, and spiculated features together with microcalcification, void vascularity, and extrathyroidal extension into the infrahyoid strap muscles. Core needle biopsy was commenced for the L1 lesion and the left large level IV lymph node. On pathologic examination, PTC with no other discernable features was found in the L1 lesion. However, malignant neoplasm was diagnosed in the lymph node specimen with a minor PTC component; the majority of the biopsied lymph node specimen revealed poorly differentiated histology different from PTC (
Fig. 2A). Immunohistochemistry was performed for the differential diagnosis. Cytokeratin, thyroid transcription factor-1 (TTF-1), and paired box gene 8 (PAX8) were negative (
Fig. 2B); only vimentin was expressed in the poorly differentiated component. The finding could be suggestive of both anaplastic transformation from PTC and collision tumor composed of PTC and sarcoma.
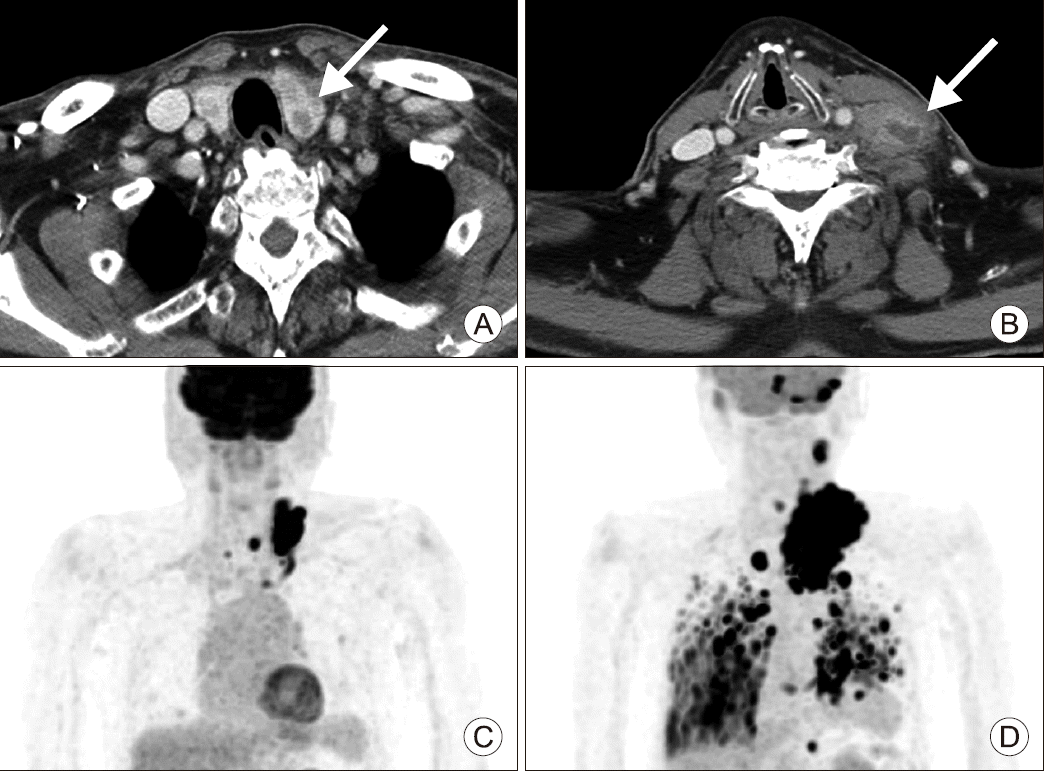 | Fig. 1(A) Computed tomography of the neck shows a large-sized mass (2×2×3.2 cm) in the left thyroid gland (arrow). (B) Computed tomography of the neck shows the left level IV lymph node with enlarged size (4.7×3.1 cm) (arrow). (C) Positron emission tomography-computed tomography (PET-CT) scan. Hypermetabolic nodule in the left thyroid lobe. Hypermetabolic enlarged lymph nodes in left neck level III, IV, right level VI and superior mediastinum. Small nodules with hypermetabolism in both lungs. (D) PET-CT scan. Local recurrence with disseminated metastasis to lymph nodes, lungs, bones, cerebellum, left adrenal gland, and cardiac RV wall. 
|
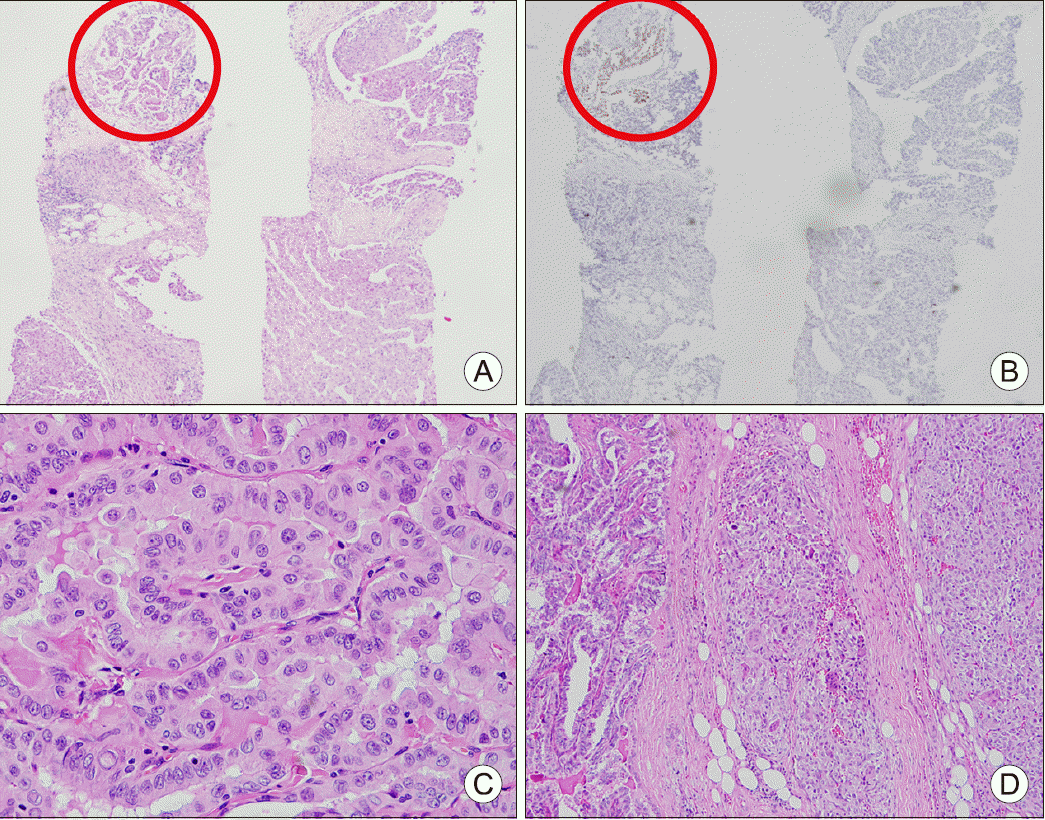 | Fig. 2Pathology finding of cervical lymph node core needle biopsy specimen. (A) The metastatic tumor composed of a minor papillary thyroid carcinoma component (left upper, red circle) and the majority of the biopsied specimen with poorly differentiated histology different from PTC; Hematoxylin & Eosin staining (H&E) ×40. (B) Immunohistochemical staining of PAX8. PAX8 was expressed only in the papillary thyroid carcinoma component (left upper, red circle) and negative in the poorly differentiated tumor component; H&E ×40. Pathology finding of surgically resected specimen. (C) Thyroid; H&E ×400. (D) Lymph node; H&E ×400. 
|
Further work-up revealed multiple, small metastatic foci in the lung, which were considered metastases of PTC at the time of diagnosis. Postoperative radioiodine ablation was planned to address the lesions (
Fig. 1C).
The initial assessment in this elderly man was PTC of the left thyroid gland, with multiple lateral cervical lymph node metastases and some extension into the anterior mediastinum; possible PTC metastasis to the lung was to be addressed with radioactive iodine therapy following surgery. A total thyroidectomy with modified radical neck dissection of the left neck (levels IIa, III, IV, Va, Vb, VI, and sternocleidomastoid muscle) and anterior superior mediastinal lymph nodes was carried out. The left recurrent laryngeal nerve was adherent to the thyroid gland; however, the tumor could be isolated without damage to the nerve. The tumor was severely adhered to the internal jugular vein, and tumor emboli were visible within the vessel, which had to be ligated and resected. The sternocleidomastoid muscle was also invaded. The carotid artery was preserved. The thyroid and the cervical specimen was gross totally resected; however, gross extranodal extension (ENE) of the tumor was present, and some tumor spillage was inevitable, resulting in R1 resection.
The patient recovered without obvious complications and was discharged on postoperative day 9. Final pathology revealed conventional PTC within the thyroid gland with an adequate safety margin (
Fig. 2C). However, PTC and ATC were co-existent in the cervical lymph node (
Fig. 2D). The majority of the metastatic tumor revealed an ATC component, and the focal area of the ATC was positive for PAX8. After review of the final pathology report and multidisciplinary tumor board discussion, postoperative radiotherapy was recommended. However, due to the advanced age and poor performance status, the patient and his family refused further adjuvant therapy. Two months postoperatively, he presented to the emergency room with dyspnea. A neck CT scan revealed a large mass in the left, previously dissected neck. A core needle biopsy under ultrasonography guidance was obtained and recurrent ATC was confirmed. Whole-body positron emission tomography-CT (PET-CT) revealed disseminated metastatic disease (
Fig. 1D). The patient refused further treatment and died of the disease 10 weeks following initial surgery.
Go to :

Discussion
More than 90% of thyroid cancers are differentiated thyroid carcinomas and most have an excellent prognosis.
15) In striking contrast, more than half of ATCs present with extensive extrathyroidal extension, lymph node metastasis, and distant disease at presentation. The disease-specific mortality for ATC exceeds well over 90%.
Although the majority of ATC originate from differentiated carcinomas within the thyroid gland, transformation of metastatic differentiated thyroid carcinomas into ATC has been reported.
3-13) Among these anecdotal case reports, four reports with a total of ten patients identified ATC transformed from PTC within the cervical lymph node (
Table 1).
6,8,9,13) Six of the patients were diagnosed with ATC in the metastatic lesions of cervical lymph nodes after thyroidectomy; in four cases, ATC was found simultaneously with PTC in the cervical lymph node metastasis, analogous to our present case. Seven case reports documented ATC in distant metastatic lesions.
3,7,10-12) The mechanism of anaplastic transformation in the well-differentiated thyroid carcinoma is still unclear. Overexpression of p53 and Ki-67 might be correlated with anaplastic transformation.
10) Further study is necessary to define this mechanism.
Table 1
Summary of current reported literatures on anaplastic transformation of PTC at cervical lymph nodes
|
Author |
Age/Sex |
Duration between identification of primary |
Post-operative |
Post-operative radioactive iodine |
Follow-up |
|
PTC and cervical ATC |
RT |
|
Sato et al.6)
|
77/M |
0 (Simultaneous) |
Not given |
Not given |
DOD |
|
Ito et al.8)
|
64/M |
3 years |
Not given |
Not given |
DOD |
|
80/F |
0 (Simultaneous) |
Not given |
Not given |
Alive |
|
51/F |
22 years |
Given |
Not given |
DOD |
|
79/M |
6 years |
Not given |
Not given |
DOC |
|
77/F |
0 (Simultaneous) |
Given |
Not given |
Alive |
|
Sung et al.9)
|
64/M |
39 years |
Not given |
Not given |
DOD (4 months) |
|
63/M |
7 years |
Not given |
Not given |
DOD (3 months) |
|
27/M |
8 years |
Not given |
Given |
DOD (4 months) |
|
Deutschmann et al.13)
|
60/M |
0 (Simultaneous) |
Given |
Given |
Alive for 1 year |
|
Present case |
85/M |
0 (Simultaneous) |
Not given (refused) |
Not given |
DOD (2 months) |

In our patient, ATC and PTC coexisted only in the core needle biopsy specimen of cervical lymph nodes. The diagnosis of ATC in lymph node biopsies was difficult, because 1) there was no evidence of ATC in the thyroid biopsy specimen, and 2) PAX8 was negative in the poorly differentiated component. PAX8 is a transcription factor that is expressed in 70-80% of ATC. In the resected lymph node specimen, PAX8 was expressed focally in the poorly differentiated component and led to the final diagnosis. Nevertheless, in retrospect, core needle biopsy provided important clinical clue regarding the aggressiveness of the otherwise indolent cancer in this patient, which might not have been possible with FNA.
In one study, long-term survival was more than five years in two out of five patients after surgery.
8) However, in this case, the tumor rapidly progressed within two months after the operation, despite a safe resection margin and adequate cervical lymphadenectomy. As shown in other cases, recurrent thyroid cancer that has converted in metastatic lesions is expected to show a poor prognosis similar to that of generalized ATC.
In summary, this case is a very rare case of a metastatic PTC that converted to ATC in the cervical lymph nodes. It is illustrative in that despite a diagnosis of differentiated thyroid cancer in the primary site, rapidly growing tumor in an elderly should raise an index of suspicion for more aggressive pathologies, including collision tumor of more aggressive nature or anaplastic transformation, as in this case. Furthermore, proper utilization of core needle biopsy may aid in earlier diagnosis of coexistent or transformed aggressive pathologies. The patients with anaplastic transformation have dismal prognosis. Because some cases showed good oncologic outcome after active treatment,
8) we recommend active ablation of tumor with surgery, radioactive iodine therapy, and external beam radiation therapy.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download