This article has been
cited by other articles in ScienceCentral.
Abstract
Chyle leakage (CL) due to lymphatic injuries is one of the rare complications that can develop after thyroidectomy. There are few studies on lymphatic embolization performed in case of CL after thyroid surgery. We report two cases of CL after thyroid surgery that were effectively treated by thoracic duct embolization. The patients had previously undergone total thyroidectomy with central compartment neck dissection with or without modified radical neck dissection. The amount of drainage from the operative site was >1000 mL per day in one patient and >500 mL per day in the other. In both cases, CL stopped after the thoracic duct embolization. Thoracic duct embolization seems to be an effective and important treatment option for CL after thyroid surgery.
Go to :

Keywords: Thoracic duct, Chyle, Thyroid gland, Thyroidectomy
Introduction
Thyroid cancer is presently the most common type of cancer in Republic of Korea.
1) The primary treatment of thyroid cancer is surgery.
2) Chyle leakage (CL) due to lymphatic injuries is one of the rare complications that can develop after thyroidectomy, with a reported incidence of 0.5-1.4% after thyroidectomy and 2-8% after neck dissections.
3) There are various options to treat CL such as low-fat diet, total parenteral nutrition (TPN), somatostatin administration, lymphatic embolization, and surgery.
There are some reports on lymphatic embolization performed in case of CL after abdominal or pelvic surgery.
4,5) However, there are few studies on lymphatic embolization performed in case of CL after thyroid surgery.
This report describes two cases of CL developing after thyroid surgery that were effectively treated by thoracic duct embolization.
Go to :

Case Reports
Case 1
A 41-year-old man had been diagnosed with papillary thyroid cancer (1.6 cm) in the left thyroid lobe during his regular medical checkup. There was no past history except old pulmonary tuberculosis. Several left level 3 metastatic lymph nodes were confirmed by preoperative ultrasonography and fine-needle aspiration biopsy. The patient underwent total thyroidectomy with central compartment neck dissection and left modified radical neck dissection. On the postoperative day (POD) 1, 1170 mL was drained through the operative site drainage tube, and 1157 mL was drained on POD 2. During this time, TPN was administered. On POD 3, interventional radiology was consulted for thoracic duct embolization. After embolization, on POD 4, the amount of drainage sharply decreased to 18 mL per day, after which the patient was discharged without any problems on POD 6 (
Table 1).
Table 1
Characteristics of patients with chyle leakage
|
Case |
Age/Sex |
Surgery |
Histologic type |
Amount of drain before the TDE (mL/day) |
The day of the TDE |
Amount of drain after the TDE (mL/day) |
The day of discharge |
|
1 |
41/M |
TT+CCND+ Lt. mRND |
PTC |
1157 |
POD 3 |
18 |
POD 6 |
|
2 |
49/F |
TT+CCND |
PTC |
563 |
POD 8 |
(−)*
|
POD 10 |

Case 2
A 49-year-old woman had been diagnosed with papillary thyroid cancer (1.8 cm) on the thyroid isthmus by ultrasonography performed due to change in voice. There was no past history except hemorrhoidectomy. She underwent total thyroidectomy with central compartment neck dissection. On PODs 1, 2, 3, and 4, 125 mL, 101 mL, 186 mL, and 132 mL, respectively, were drained through the operative site drainage tube. During this time, TPN was administered. On POD 5, the amount of CL increased, 639 mL was drained, and we started to administer somatostatin. On PODs 6 and 7, 485 mL and 563 mL, respectively, were drained. On POD 8, the patient was referred to interventional radiology for thoracic duct embolization. After the procedure, the drainage tube was accidentally removed, but there was no swelling at the operative site after embolization. The patient started diet and was discharged without any problems on POD 10 (
Table 1).
Technique
We performed thoracic duct embolization with the following technique. The patient lay on the fluoroscopy table in supine position during the procedure. A high- frequency (7-15 MHz) linear transducer (HD11XE
®; Philips Medical Solutions, Mountain View, CA, USA) was used to guide a 25-G fine needle into a lymph node located in the upper thigh (
Fig. 1). Lipiodol (Guerbet, Aulnay-sous-Bois, France) was manually injected at a rate of 1 mL/min under fluoroscopic and/or C-arm computed tomography guidance (DynaCT; Siemens, Erlangen, Germany). Intranodal Lipiodol lymphangiography was performed until the Lipiodol penetrated the retroperitoneal lymphatic vessels, passed through the cisterna chyli, and finally reached the thoracic duct to reveal the leakage site. Extravasation of Lipiodol was identified at the upper thoracic level where the extravasated Lipiodol was evacuated through the operative site drain (
Fig. 2). Transabdominal puncture of the cisterna chyli was performed using a 22-G needle under fluoroscopic guidance (
Fig. 3). A 2.0-Fr microcatheter was co-axially advanced into the thoracic duct. A detachable coil and glue mixture (N-butyl cyanoacrylate [B. Braun Melsungen AG, Melsungen, Germany] diluted in Lipiodol at a ratio of 1:1) were used to embolize the distal thoracic duct just below the leakage site (
Fig. 4). On the day after the procedure, follow-up chest X-ray was performed (
Fig. 5). The study protocol was approved by the Institutional Review Board of Ajou Medical Center (AJIRB-MED- MDB-19-090). Informed consent was not obtained because of the retrospective chart review study design and use of only deidentified clinicopathological information.
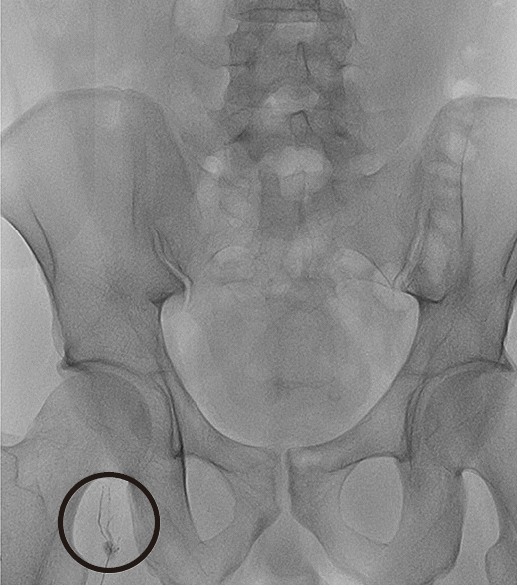 | Fig. 1A single lymph node in the right thigh was punctured using a 25-G needle. 
|
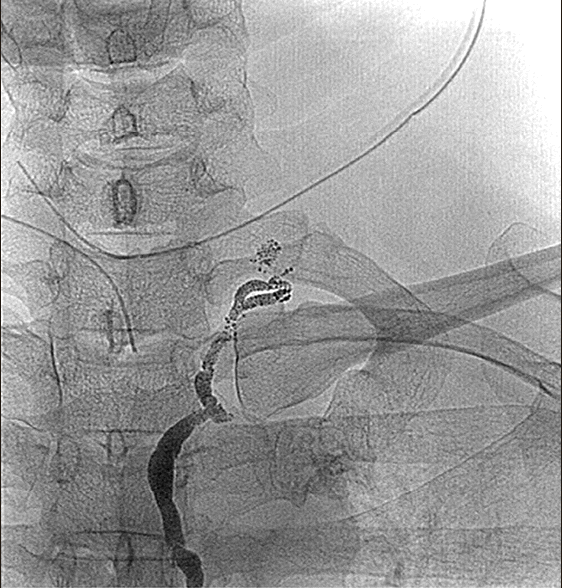 | Fig. 2The leakage site was detected near the cervical portion of the thoracic duct. 
|
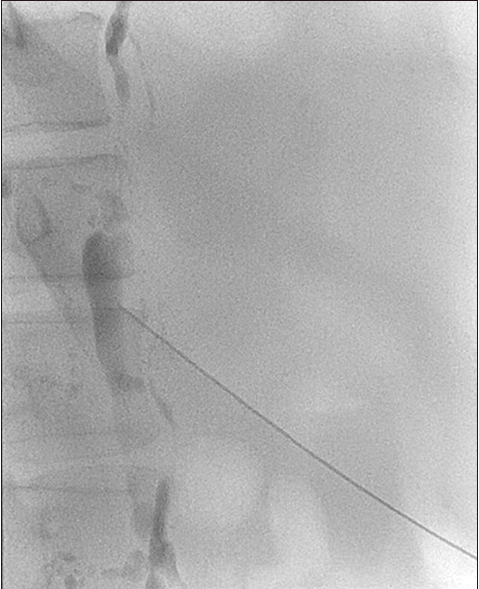 | Fig. 3The cisterna chyli was accessed using a 22-G needle, followed by co-axial delivery of a 2.0-Fr microcatheter into the thoracic duct. 
|
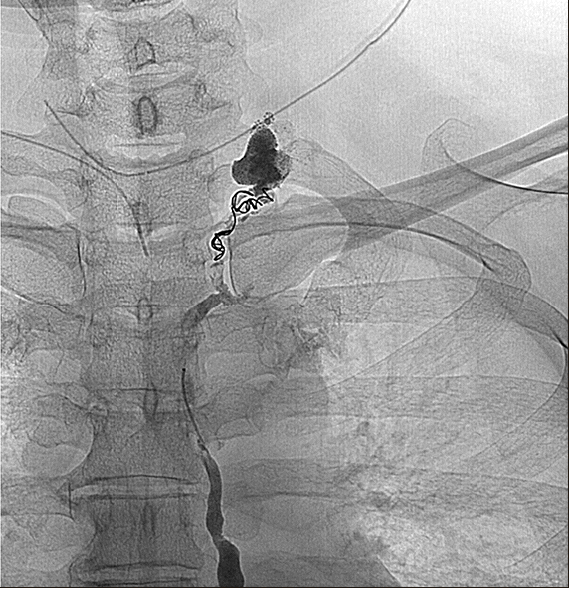 | Fig. 4Coil and glue were used to embolize the distal thoracic duct just below site of leakage. 
|
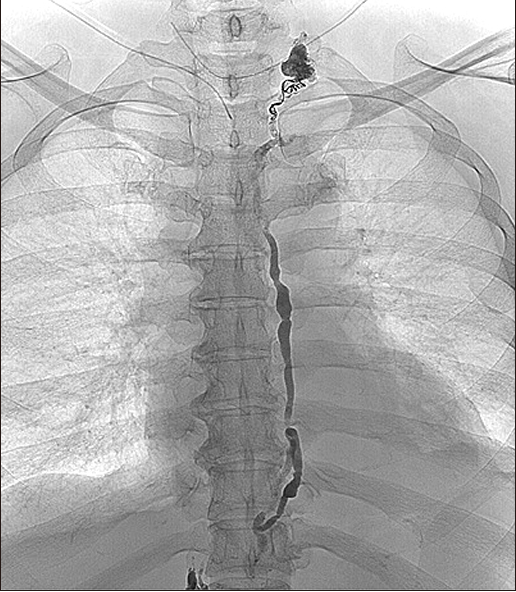 | Fig. 5
|
Go to :

Discussion
The thoracic duct originates from the cisterna chyli. Within the abdomen, the thoracic duct ascends along the anterior surface of the lumbar vertebra. It continues to ascend through the thorax, entering the neck. At the neck, as the most common anatomy, it turns inferiorly and terminates at 1 cm of the confluence of the left internal jugular vein (IJV) and left subclavian vein.
3) The thoracic duct is lined by of a thin wall of cloudy, white color. It is the largest lymphatic vessel in the body and the main trajectory of the lower and upper left body. All the body’s lymphatic fluid, except that in the upper right body, enters the thoracic duct.
Unintentional damage to the thoracic duct can occur during neck surgery. There are several causes of thoracic duct injury: proximity to the IJV, thin vessel wall,
6) prior irradiation,
7) and presence of metastatic lesions at the confluence of the IJV and subclavian vein.
8)
CL can cause various problems. Delayed wound healing, infection, or wound breakdown with fistula formation can result from CL.
3) Dehydration, electrolyte disturbances, malnutrition, and immunosuppression can also be caused by CL.
6,9) CL can result in chylothorax, which presents clinically with shortness of breath, tachypnea, and chest pain.
3)
Treatment of CL includes conservative measures such as hydration, low-fat diet, TPN, pressure dressings, suction drainage, and somatostatin administration in case of low-output CL (<500 mL/day).
3) The patient is usually required to tolerate a long hospitalization period during this time. High-output CL (>500 mL/day) often responds unsatisfactorily to conservative management alone and requires surgical intervention. However, with surgical re-exploration, local inflammation from extravasated chyle can make thoracic duct identification difficult.
3) Furthermore, the patient has to endure the burden of reoperation. Among the various options, lymphatic embolization possesses an advantage, owing to its minimally invasive nature. Because the treatment effect appears almost immediately, prolonged hospitalization is not required. The complications from reoperation can also be avoided. Chen and Itkin reported that the overall success rate of thoracic duct embolization in traumatic chylothorax was 91%.
10) However, little is known about the success rate of thoracic duct embolization in cases of CL after thyroid surgery.
In our two cases, CL stopped after the procedure. The patients returned to normal diet and were discharged without any problems. In both cases, thoracic duct embolization was an effective treatment option.
Although more studies on thoracic duct embolization for CL after thyroid surgery are needed, it seems to be an effective treatment option for CL after thyroid surgery.
Go to :





 PDF
PDF Citation
Citation Print
Print








 XML Download
XML Download