Abstract
Background and Objectives
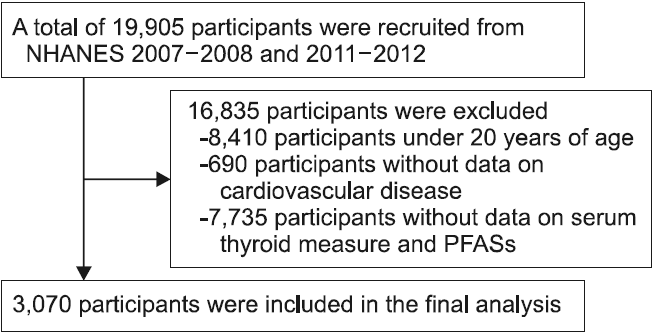
Materials and Methods
Results
Aknowledgments
REFERENCES
Table 1
| Characteristic | N (%) | Thyroid measures | Perfluoroalkyl substances | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| TSH (μlU/mL) | Total T4 (ng/dL) | Total T3 (ng/dL) | FreeT4 (ng/dL) | PFOA (μg/L) | PFOS (μg/L) | PFHxS (μg/L) | Me-PFOSA-AcOH (μg/L) | PFNA (μg/L) | PFDeA (μg/L) | PFUA (μg/L) | ||
|
|
|
|
|
|
|
|
|
|
|
|
||
| M (IQR) | M (IQR) | M (IQR) | M (IQR) | M (IQR) | M (IQR) | M (IQR) | M (IQR) | M (IQR) | M (IQR) | M (IQR) | ||
| %>LOD | 3070 | - | - | - | - | 99.6 | 99.7 | 98.6 | 54.6 | 99.3 | 71 | 41.5 |
| Total | 3070 | 1.55 [1.06;2.30] | 7.74 [6.88;8.80] | 112 [99;126] | 0.80 [0.70;0.90] | 3.00 [1.90;4.80] | 10.40 [5.80;18.00] | 1.60 [0.87;2.80] | 0.20 [0.14;0.40] | 1.07 [0.74;1.64] | 0.25 [0.14;0.40] | 0.14 [0.14;0.29] |
| Age | ||||||||||||
| 20-50 | 1671 (54.4%) | 1.44 [1.01;2.09] | 7.66 [6.80;8.69] | 116 [105;131] | 0.80 [0.70;0.89] | 2.64 [1.71;4.39] | 8.40 [4.80;14.10] | 1.28 [0.70;2.50] | 0.14 [0.14;0.30] | 0.98 [0.66;1.54] | 0.22 [0.14;0.40] | 0.14 [0.14;0.23] |
| 51-65 | 738 (24.0%) | 1.60*** [1.12;2.37] | 7.75*** [6.86;8.80] | 110*** [98;124] | 0.80*** [0.70;0.90] | 3.30*** [2.10;5.16] | 11.65*** [7.02;20.50] | 1.73*** [1.00;2.86] | 0.20*** [0.14;0.40] | 1.15*** [0.82;1.72] | 0.28** [0.14;0.40] | 0.14*** [0.14;0.30] |
| ≥66 | 661 (21.5%) | 1.77*** [1.18;2.71] | 8.00*** [7.02;9.08] | 104*** [90;114] | 0.80*** [0.70;0.90] | 3.50*** [2.38;5.40] | 15.60*** [9.30;25.00] | 2.15*** [1.30;3.51] | 0.30*** [0.14;0.60] | 1.15*** [0.81;1.80] | 0.28** [0.14;0.48] | 0.14*** [0.14;0.30] |
| Sex | ||||||||||||
| Male | 1586 (51.7%) | 1.52 [1.06;2.27] | 7.60*** [6.70;8.60] | 113.*** [102;127] | 0.80 [0.70;0.90] | 3.46*** [2.26;5.40] | 12.70*** [7.75;21.30] | 2.10*** [1.23;3.40] | 0.2 [0.14;0.40] | 1.16*** [0.82;1.72] | 0.29*** [0.14;0.40] | 0.14** [0.14;0.30] |
| Female | 1484 (48.3%) | 1.58 [1.07;2.32] | 7.97 [7.08;9.00] | 110 [98;124] | 0.80 [0.70;0.90] | 2.57 [1.56;4.20] | 8.10 [4.44;13.75] | 1.1 [0.61;2.02] | 0.18 [0.14;0.36] | 0.98 [0.66;1.48] | 0.21 [0.14;0.40] | 0.14 [0.14;0.26] |
| Race | ||||||||||||
| Mexican American | 427 (13.9%) | 1.55 [1.06;2.28] | 8.06 [7.10;9.10] | 118 [105;132] | 0.80 [0.70;0.90] | 9.10 [5.64;14.65] | 1.40 [0.80;2.50] | 0.14 [0.14;0.30] | 0.95 [0.69;1.46] | 0.20 [0.14;0.38] | 0.14 [0.14;0.14] | 9.10 [5.64;14.65] |
| Other Hispanic | 349 (11.4%) | 1.59*** [1.06;2.30] | 7.90*** [7.10;9.00] | 116*** [104;129] | 0.80*** [0.70;0.90] | 8.30*** [4.77;13.40] | 1.40*** [0.66;2.40] | 0.14*** [0.14;0.28] | 1.04*** [0.68;1.51] | 0.20*** [0.14;0.39] | 0.14*** [0.14;0.27] | 8.30*** [4.77;13.40] |
| Non-Hispanic White | 1263 (41.1%) | 1.72*** [1.19;2.55] | 7.60*** [6.70;8.57] | 110*** [97;125] | 0.80*** [0.70;0.89] | 11.00*** [6.46;18.40] | 1.80*** [1.07;3.10] | 0.30*** [0.14;0.50] | 1.03*** [0.73;1.52] | 0.21*** [0.14;0.40] | 0.14*** [0.14;0.20] | 11.00*** [6.46;18.40] |
| Non-Hispanic Black | 675 (22.0%) | 1.29*** [0.88;1.88] | 7.75*** [6.83;8.80] | 111*** [98;123] | 0.80*** [0.70;0.89] | 12.00*** [6.19;23.10] | 1.78*** [0.90;3.06] | 0.20*** [0.14;0.42] | 1.23*** [0.82;1.81] | 0.30*** [0.17;0.50] | 0.18*** [0.14;0.36] | 12.00*** [6.19;23.10] |
| Other race | 356 (11.6%) | 1.52*** [1.03;2.14] | 7.80*** [6.96;8.89] | 111*** [101;123] | 0.85*** [0.77;0.94] | 9.41*** [4.86;16.25] | 1.12*** [0.69;1.90] | 0.14*** [0.14;0.30] | 1.19*** [0.70;1.98] | 0.36*** [0.19;0.69] | 0.30*** [0.14;0.80] | 9.41*** [4.86;16.25] |
| BMI (kg/m2) | ||||||||||||
| <25 | 1951 (64.4%) | 1.49*** [1.02;2.20] | 7.61*** [6.80;8.65] | 111*** [99;125] | 0.80 [0.70;0.90] | 3.01 [1.98;4.90] | 10.80* [6.11;18.20] | 1.60* [0.90;2.90] | 0.20 [0.14;0.40] | 1.07 [0.74;1.64] | 0.28*** [0.14;0.40] | 0.14*** [0.14;0.30] |
| ≥25 | 1079 (35.6%) | 1.64 [1.15;2.43] | 7.97 [7.00;9.04] | 114 [101;128] | 0.80 [0.70;0.90] | 3.00 [1.82;4.78] | 9.74 [5.54;17.85] | 1.50 [0.80;2.68] | 0.19 [0.14;0.35] | 1.07 [0.74;1.58] | 0.21 [0.14;0.40] | 0.14 [0.14;0.20] |
| Smoking | ||||||||||||
| Never | 1687 (55.0%) | 1.60** [1.09;2.38] | 7.75 [6.90;8.80] | 112 [100;125] | 0.80 [0.70;0.90] | 2.81*** [1.80;4.60] | 9.92** [5.60;17.25] | 1.40*** [0.78;2.60] | 0.16*** [0.14;0.30] | 1.07 [0.73;1.64] | 0.26* [0.14;0.40] | 0.14*** [0.14;0.30] |
| Smoker | 1380 (45.0%) | 1.48 [1.03;2.22] | 7.71 [6.80;8.76] | 112 [98;127] | 0.80 [0.70;0.90] | 3.20 [2.08;5.10] | 10.80 [6.20;18.90] | 1.80 [1.00;3.00] | 0.20 [0.14;0.41] | 1.07 [0.74;1.64] | 0.24 [0.14;0.40] | 0.14 [0.14;0.23] |
| Alcohol | ||||||||||||
| Never | 766 (27.3%) | 1.60 [1.08;2.43] | 8.06*** [7.11;9.10] | 111 [98;125] | 0.80* [0.70;0.90] | 2.90** [1.71;4.70] | 9.80** [5.59;17.70] | 1.30*** [0.70;2.41] | 0.20 [0.14;0.40] | 1.07 [0.67;1.64] | 0.23 [0.14;0.40] | 0.14 [0.14;0.30] |
| Drinker | 2042 (72.7%) | 1.53 [1.06;2.26] | 7.60 [6.72;8.68] | 112 [100;126] | 0.80 [0.70;0.90] | 3.11 [2.05;4.93] | 10.70 [6.15;18.40] | 1.71 [0.98;3.00] | 0.20 [0.14;0.40] | 1.07 [0.74;1.64] | 0.26 [0.14;0.40] | 0.14 [0.14;0.26] |
| Thyroid autoantibody status | ||||||||||||
| Negative | 2689 (88.1%) | 1.50*** [1.05;2.18] | 7.73 [6.87;8.77] | 112 [99;126] | 0.80** [0.70;0.90] | 3.05 [1.93;4.85] | 10.30 [5.91;18.10] | 1.60 [0.88;2.80] | 0.20 [0.14;0.40] | 1.07 [0.74;1.64] | 0.26 [0.14;0.40] | 0.14 [0.14;0.29] |
| Positive | 362 (11.9%) | 2.01 [1.22;3.23] | 7.80 [6.90;9.02] | 112 [100;127] | 0.80 [0.70;0.89] | 2.80 [1.80;4.70] | 10.85 [5.50;17.30] | 1.50 [0.80;2.70] | 0.16 [0.14;0.38] | 1.07 [0.73;1.64] | 0.21 [0.14;0.40] | 0.14 [0.14;0.30] |
BMI: body mass index, IQR: interquartile range, LOD: limit of detection, M: median, Me-PFOSA-AcOH: 2-(N-methyl-perfluorooctane sulfonamido) acetic acid, PFDeA: perfluorodecanoate, PFHxS: perfluorohexane sulfonate, PFNA: perfluorononanoate, PFOA: perfluorooctanoate, PFOS: perfluorooctane sulfonate, PFUA: perfluoroundecanoate, TSH: thyroid-stimulating hormone
Table 2
| PFASs | PFOA | PFOS | PFHxS | Me-PFOSA-AcOH | PFNA | PFDeA | PFUA |
|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
| correlation coefficients | correlation coefficients | correlation coefficients | correlation coefficients | correlation coefficients | correlation coefficients | correlation coefficients | |
| PFOA | 1 | 0.686** | 0.636** | 0.309** | 0.634** | 0.444** | 0.134** |
| PFOS | 1 | 0.689** | 0.339** | 0.670** | 0.578** | 0.337** | |
| PFHxS | 1 | 0.252** | 0.449** | 0.292** | 0.118** | ||
| Me-PFOSA-AcOH | 1 | 0.166** | 0.100** | -0.035 | |||
| PFNA | 1 | 0.765** | 0.546** | ||||
| PFDeA | 1 | 0.686** | |||||
| PFUA | 1 |
Table 3
| PFASs | TSH | Total T4 | Total T3 | Free T4 | TPO antibodies* | Tg antibodies* |
|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| PFOA | 0.012 | −0.158 | 0.122 | −0.014 | −0.073 | 2.438 |
| (−0.023, 0.047) | (−0.244, −0.072) | (−1.049, 1.294) | (−0.022, −0.007) | (−4.525, 4.379) | (−1.792, 6.667) | |
| PFOS | −0.010 | −0.083 | −0.812 | −0.005 | 2.07 | 3.036 |
| (−0.04, 0.019) | (−0.155, −0.012) | (−1.785, 0.161) | (−0.012, 0.001) | (−1.624, 5.764) | (−0.477, 6.549) | |
| PFHxS | −0.003 | −0.015 | 0.913 | −0.004 | −0.011 | 2.732 |
| (−0.03, 0.024) | (−0.081, 0.051) | (0.015, 1.812) | (−0.01, 0.002) | (−3.423, 3.4) | (−0.514, 5.978) | |
| Me-PFOSA-AcOH | 0.019 | −0.081 | −0.462 | −0.007 | −0.120 | 1.829 |
| (−0.014, 0.053) | (−0.164, 0.001) | (−1.584, 0.66) | (−0.014, 0.001) | (−4.387, 4.147) | (−2.219, 5.877) | |
| PFNA | −0.046 | −0.048 | −0.316 | −0.002 | −0.603 | 3.828 |
| (−0.083, −0.009) | (−0.139, 0.043) | (−1.559, 0.927) | (−0.011, 0.006) | (−5.331, 4.124) | (−0.669, 8.324) | |
| PFDeA | −0.04 | 0.003 | −1.162 | −0.001 | −0.032 | 1.456 |
| (−0.078, −0.002) | (−0.09, 0.096) | (−2.425, 0.101) | (−0.01, 0.007) | (−4.84, 4.776) | (−3.114, 6.026) | |
| PFUA | −0.033 | 0.055 | −0.986 | 0.007 | 0.126 | −0.692 |
| (−0.074, 0.007) | (−0.044, 0.154) | (−2.33, 0.358) | (−0.002, 0.016) | (−4.989, 5.241) | (−5.555, 4.172) |
*Adjusted for sex, age, race/ethnicity, body mass index, smoking status, alcohol consumption, and urinary iodine concentration.
Adjusted for sex, age, race/ethnicity, body mass index, smoking status, alcohol consumption, urinary iodine concentration, thyroglobulin antibody and thyroperoxidase antibody.
CI: confidence interval, Me-PFOSA-AcOH: 2-(N-methyl-perfluorooctane sulfonamido) acetic acid, PFASs: perfluoroalkyl substances, PFDeA: perfluorodecanoate, PFHxS: perfluorohexane sulfonate, PFNA: perfluorononanoate, PFOA: perfluorooctanoate, PFOS:perfluorooctane sulfonate, PFUA: perfluoroundecanoate, TPO: thyroperoxidase, TSH: thyroid-stimulating hormone
Table 4
| PC 1 | PC 2 | |
|---|---|---|
| PFOA | 0.831* | 0.277 |
| PFOS | 0.776* | 0.481 |
| PFHxS | 0.834* | 0.157 |
| Me-PFOSA-AcOH | 0.569* | −0.117 |
| PFNA | 0.521* | 0.723* |
| PFDeA | 0.199 | 0.910* |
| PFUA | −0.076 | 0.919* |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download