INTRODUCTION
Human immunodeficiency virus-1 (HIV) is the causative agent of Acquired immunodeficiency syndrome (AIDS). It is an infectious disease, 37 million people have been infected and about 1 million people have died every year. The antiretroviral combination therapy highly active antiretroviral therapy (HAART) has greatly reduced the virus load and increased the survival rate, but the development of a novel therapy is urgently required because of the adverse side effects of the drug and the HIV-1 drug-resistant mutations. A study demonstrated that the functions of the HIV-1 viral protein Nef are defective in the HIV-1 infected long-term non-progressor (LTNP). Thus, new therapeutic strategies have been spotlighted to eliminate virulence by targeting Nef.
Nef is a small 27-35 kDa viral protein with myristoylation at the N-terminal, and attaches itself to the membrane lipid bilayer (1). The papers published in Science (2)and NEJM (3) in 1995 suggested first that nef gene is an important virulence-inducing factor, as it was confirmed that the nef gene was commonly defective in HIV-1 isolates of LTNPs through an analysis of the HIV genome of LTNPs (3). The Sydney Blood Bank Cohort (SBBC) study conducted in 2007, it was supported that the correlation between the progression of AIDS and defect of nef gene in LTNPs and slow progressors (SPs) was highly consistent (4). Also, the nef defective virus can be transmitted by sexual relations, but the viral concentration remained very low in patients infected by this virus. Numerous studies have determined the virulence mechanism underlying the molecular biology of Nef, as Nef down-regulated surface expression of CD4 and major histocompatibility complex class I (MHC-I/II), increased viral infectivity, disturbed actin dynamics and vesicle trafficking, and induced virus transcription. Comparably, these functions in the Nef of simian immunodeficiency virus (SIV) were significantly lower than those of HIV-1, resulting in weak virulence. These results revealed that the molecular biological mechanism of Nef was one of main cause of inducing virulence (1, 5, 6).
This study aims to introduce the virulence-inducing mechanism of the HIV-1 Nef and propose new therapeutic strategies against HIV-1 through Nef targeting.
Go to : 
BODY
Nef virulence related mechanism
CD4 down-regulation
CD4 is a T-cell membrane protein which binds to HIV-1 gp120 to facilitate HIV-1 infection, whereas, CD4 expression is diminished by HIV-1 infection. It was defined that the down-regulation of CD4 was caused by Nef expression after HIV-1 infection in an initial study. The detail mechanism of CD4 down-regulation was revealed that CD4 on the surface membrane was internalized and degraded through the endosomal/lysosomal pathway. It has been known that the adapter protein 2 (AP-2), which is a clathrin-associated adaptor protein acting on endocytosis, plays an important role in the inhibition of CD4 expression by Nef (7). It may be possible that the CD4 down-regulation induced by Nef blocks super-infection and promotes viral transmission due to facilitating virion release during budding out. Furthermore, it is more worthy note that CD4-down regulation by Nef is closely associated to host immune dysfunction leading to AIDS. On that basis, it might be possible to reduce the virulence by blocking the Nef-induced CD4-down regulation.
MHC-I down-regulation
It may be possible that cytotoxic T lymphocytes (CTL) response eliminates HIV-1 infected cells. The CTL response, however, is not efficient in HIV-1 infected individuals harboring reservoir cells infected with latent virus. Nef together with MHC-I from the plasma membrane into the trans Golgi network (TGN) by binding to PACS-2, inhibited MHC-I to move out to the cell membrane, and finally prevented expose of MHC-I on the surface membrane (8). Another study showed that Nef promoted an interaction between the cytoplasmic tail of MHC-I and AP-2 to inhibit membrane expression. This mechanism of Nef may disable CTL response to eliminate HIV-1 infected cells, thereby promoting virulence by increasing the proliferation and concentration of the virus and disturbing the immune system.
T cell activation signaling
Proliferation of HIV-1 is closely related with the T-cell activation signals including transcription factors of NF-κb, AP1 and NFAT which are activated by Nef expression. Nef-induced activation of these transcriptional factors also promote the initiation of viral transcription leading to viral proliferation (9). Otherwise, Nef binds to the SH3 domain of Pak2 or Src Family Kinases (Hck, Lyn and c-Src) and activates them, which strongly promotes activation signals of T-cells alone without T-cell activation linked to CD3- TCR (T-cell receptor).
CD3 down-regulation
The Nef of simian immunodeficiency virus (SIVsm) which infects sooty mangabey monkey down-regulates the CD3 expression on surface of T-cell. As the CD3 down-regulation by SIV-Nef suppresses T cell activation associated to viral replication, SIV showed limited viral proliferation and dose not induce virulence in monkeys (10). In contrast, it was known that Nef of HIV-1 and SIVcpz (SIV chimpanzee) did not down-regulate the CD3 expression. However, the recent study confirmed that CD3 is also down-regulated in cells persistently infected by HIV-1 over a long period of time, which appears a close relationship between HIV-1 virulence linked to impaired immune function and persistent infection.
Cell mobility inhibition
It has been reported that HIV-1 Nef inhibits the extracellular secretion of the stromal derived factor (SDF) to inhibit T cell mobility, followed by a decreased immunological activity of T cells. The Nef-expressed cell lost mobility despite the migration signal stimulus, which is thought to be due to inhibition of the actin dynamics required for cell migration. One study reported that the interaction between Nef and Pak2 inhibits cofilin activity, resulting in dissociation from filamentous actin to globular actin which cause interruption of cell movement (11). In another study with an in-vivo mouse model, the actin dynamics of the Nef-expressed T cells were disrupted despite the T cell chemotaxis stimuli, and the cells failed to penetrate the immune tissues. These studies provided evidences that Nef inhibit T-cell chemotaxis for immunological T-cell function and induces immune deficiency (12).
Vesicle & membrane trafficking disturbance
In T cells expressed with HIV-1 Nef, membrane hypermobility was observed as if the activation of endocytosis and exocytosis seen in activated T cells. Nef induces membrane trafficking modification to promote secretory activity (13). More surprisingly, such abnormality of membrane trafficking was also observed in neighboring non-infected cells, which was understood that Nef could induce the same membrane trafficking disturbance through cell to cell membrane contacting to neighboring cells.
T cell receptor signal disturbance
The T cell receptor (TCR) signal triggered by TCR-CD3 activation, express the IL-2 chemokine through the Lck > Zap70 > PLC > IP3 > IP3 receptor > calcium release > NFAT/NF-κb > IL-2. However, Nef expression activates these signals alone through endocytosis of Lck and LAT, which are responsible for the TCR signal, into the intracellular compartment (14). Moreover, Nef binds directly to the IP3 receptors on the endoplasmic reticulum (ER) membrane and activates them to release calcium in the ER, triggering T cell activation signals such as NFAT (14).
Increase of viral infectivity
Several evidences that Nef increases HIV-1 infectivity are already well known, but various hypotheses concerning its mechanism have been suggested. First, a study reported that the cortical actin of virion-infected cells inhibited the transfer of the viral nuclear capsid into the cytoplasm, and that Nef inhibited this cortical actin to maintain viral core penetration. Second, a hypothesis exists that Nef inhibits the degradation of the penetrated core through the proteasome to help viral infection. Third, it is known that a certain unknown protein on the virion membrane, which originated from the cell membrane at budding, blocked virion re-infection. The results of other studies showed that Nef eliminates proteins that block virion infection and then promotes second round infection of virion produced from earlier infected cells. Recently, a study reported a membrane protein called SERINC5 as the intra-virion protein that blocks re-infection as suggested in the above hypothesis (15). SERINC5 is incorporated into the virion membrane and blocks re-infection, and Nef inhibits SERINC5 expression and limits its incorporation into the virion to promote virion re-infection. This function was proven to be the mechanism by Nef-induced endosomal/lysosomal degradation.
Other immune disturbances
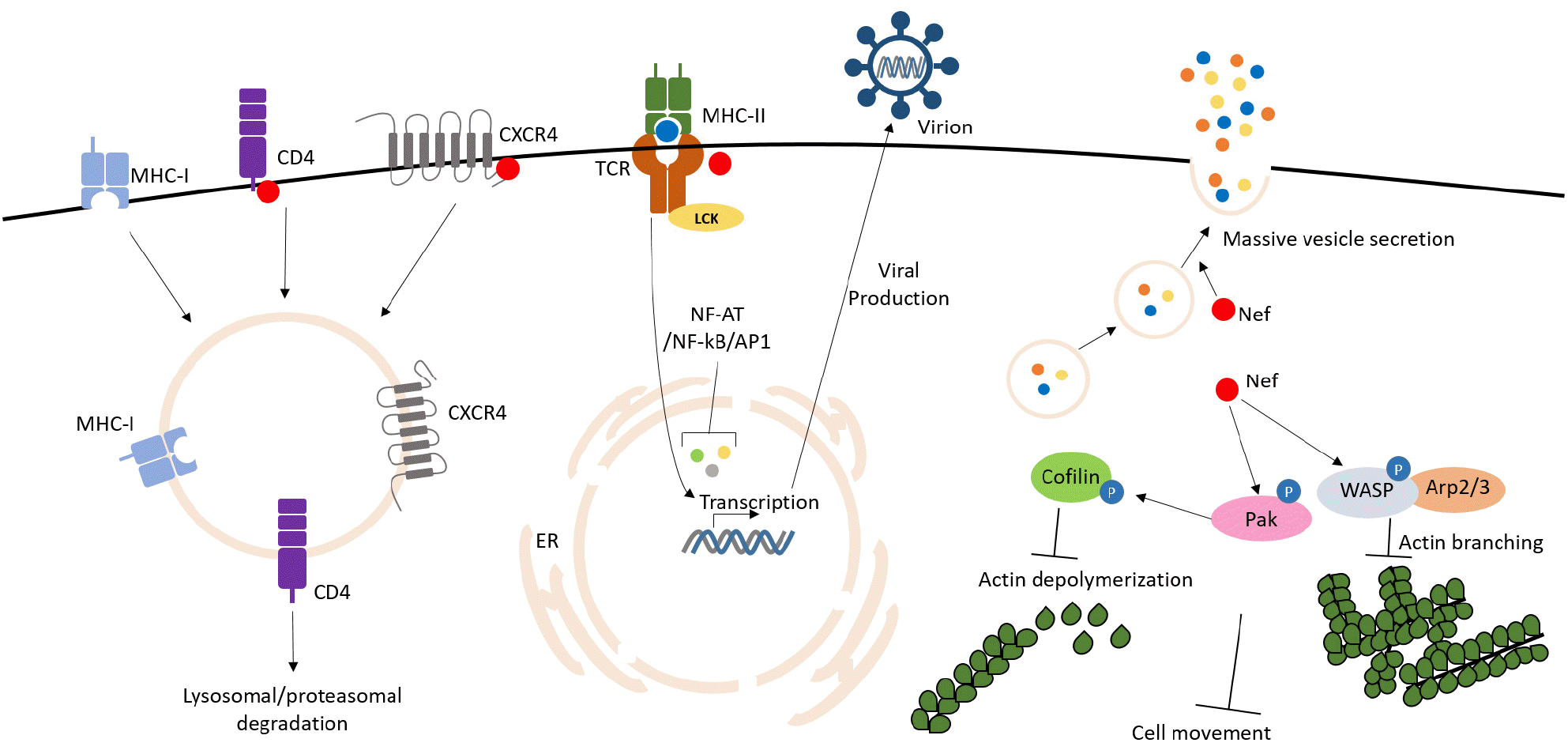
Furthermore, Nef is known to down-regulate CD62L, an adhesion molecule that promotes immune cell circulation from the vascular endothelium to the peripheral lymph node (16). In addition, Nef reduced the membrane expression of negative immune modulator CTLA-4, and down-regulated CD28 and CXCR4/CCR5 (17). These functions suggest that Nef is the core factor in the induction of viral virulence by the inhibition of efficient immune responses of immune cells (Fig. 1).
Therapeutic aspects of Nef
As Nef-induced molecular biological mechanisms related to the diseases progression have been revealed, it has been emerged as new targets to block such mechanisms in order to inhibit or at least delay the progression of diseases. Therefore, many researchers have tried to discover compounds that block the binding between Nef and intracellular factors.
D1 was discovered by binding to the PxxPxxP motif of SH3, a hydrophobic pocket of Nef, through a virtual screening based on a protein-protein interaction assay (18). This partially blocked binding with Nef-MHC-I, after which DLC27 and its derivatives were developed through an additional screening for substructures to increase the bonding ability. However, it is no longer being developed due to solubility and toxicity problems, and other inhibitory effect on Nef function is not known.
In another study, inhibitors of several cellular kinases were reported as inhibitors of MHC-I down-regulation on the basis that MHC-I down-regulation by Nef is proceeded through the Nef > SFK > Zap70 >PI3K signal transduction system (Nef-associated multi-kinase complex). PI3K inhibitors such as PI-103 and PKI-23 were discovered and suggested for the availability.
Another study developed a compound capable of inhibiting interaction between Nef and the kinase, namely, USCS15A derivative ‘2c’, a metabolite of Streptomyces, which was originally developed as a substance that blocks binding between PxxP, which acts on the binding with Sam68-c-src, and the src-SH3 domain. However, 2c is known to inhibit binding with Nef-Hck/Lyn/c-Src/SFKS in various ways, and is expected to block various virulence mechanisms caused by Nef. In addition, it was found that 2c inhibited Nef-induced viral infectivity, but it was possible only in scores of several μM concentration, suggesting the possibility of an off-target effect (nonspecific effect); furthermore, 2c was found to be absolutely ineffective in inhibiting the CD4 down-regulation caused by Nef.
Another study discovered the 4-amino-diphenylfuranopyrimidine (DFP) scaffold by developing a high-throughput screen assay using the kinase activity of Nef-dependent Hck. It inhibited Hck activity well under the presence of Nef. Also, DFP-based compounds were observed to inhibit the promotion of Nef-dependent HIV-1 proliferation at several μM. Although it also inhibited Nef-dependent SFK activity, it is unknown whether the SFK inhibition mechanism is related to the inhibition of HIV-1 replication. However, these strategies tried to develop compounds that control Nef-dependent host kinase activity, but much more concern is needed because side effects developed as a result of inhibiting the original functions of the host kinases.
Another study developed a diphenylpyrazolo compound (B9), which selectively inhibits Nef-dependent Hck activity to a significant extent through a mass screening of 220,000 compounds in the Nef/Hck assay (18). It showed more efficient inhibitory effect on Hck activity combined with Nef compared to Hck alone, and inhibited Nef-dependent HIV-1 proliferation at several hundred nM, as well as inhibiting SFK activity. It has been reported that, unlike other existing kinase inhibitors, B9 binds directly to Nef; and, according to several X-ray crystal structure analyses, it binds to interfaces of two Nef. Recently, it has been revealed that there are many relationships between Nef activity and its dimerization, and that, in particular, dimerization of Nef is important in Nef-induced Hck activity (Fig. 2).
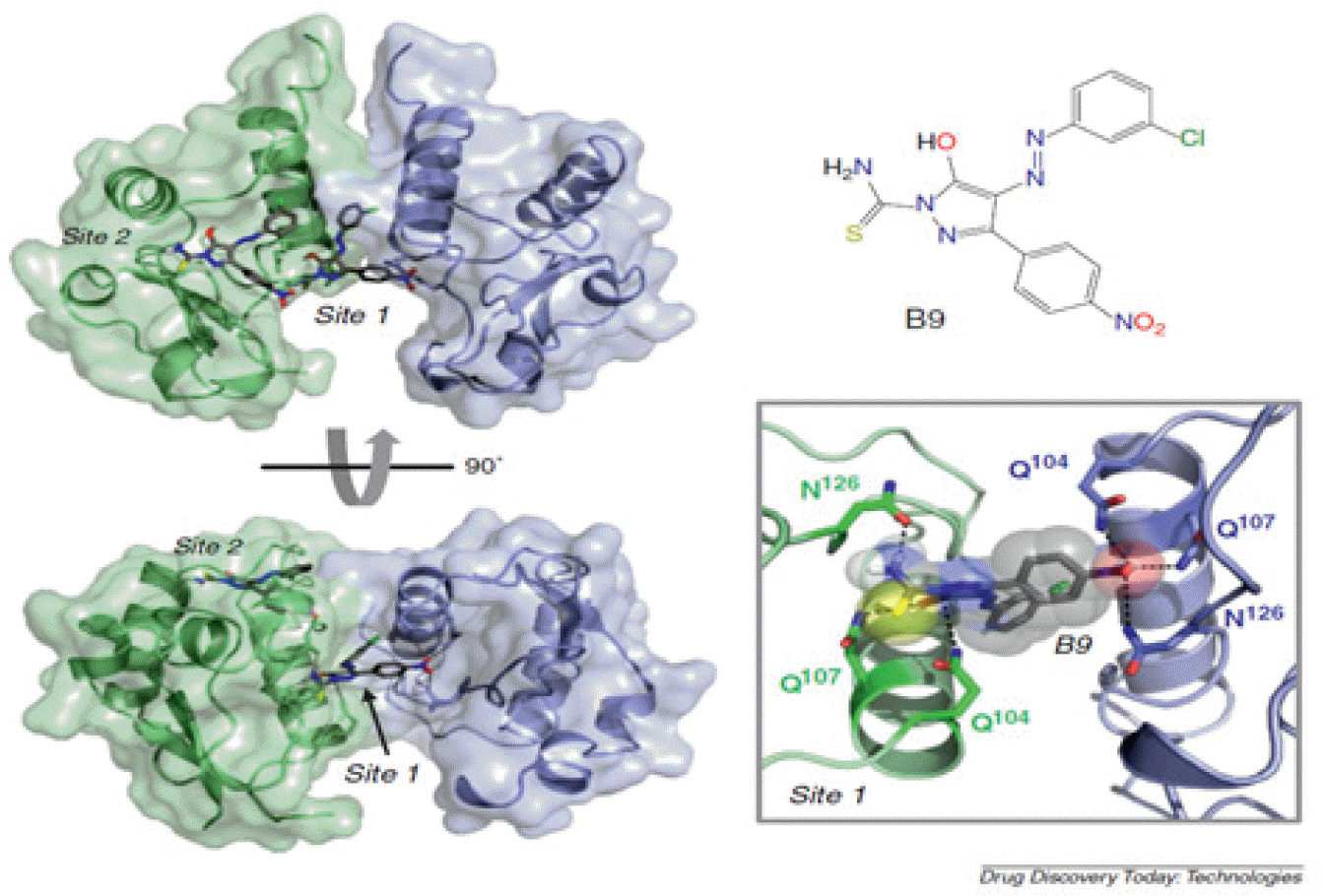 | Fig. 2Diphenylpyrazolo compound B9 inhibits Nef function (18).B9 antagonists Nef by binding to residues Q104, Q107 and N126 in interface for Nef dimerization. |
The KNIH has conducted studies aimed at discovering host proteins that interact with Nef in order to understand the fundamental virulence mechanism of Nef and develop a novel therapeutic strategy capable of inducing the asymptomatic infection. Researchers discovered about 60 kinds of Nef-binding host proteins using yeast two-hybrid screening (Fig. 3). We identified the following proteins: ACTB (NM_001101), LTB (NM_009588), AP2A2 (NM_005968), ATAD3A (NM_018188), TNK2 (NM_001010938), EIF3F (NM_003754), HJURP (NM_018410), BAG5 (NM_001015049), HNRNPD (NM_001003810), ATAD5 (NM_024857), GAPDH (NM_002046), and CNN3 (NM_001839) and studied certain proteins which are expected to be related to HIV-1 virulence. Among these, TNK2, also called ACK (Activated Cdc42-associated kinase), is closely related to actin dynamics regulatory factor Cdc42 activity. We are conducting research to verify whether Nef disrupts the actin dynamics of host cell by binding to TNK2, followed by down-regulation of the immune receptors such as CD4, MHC, and CXCR4.
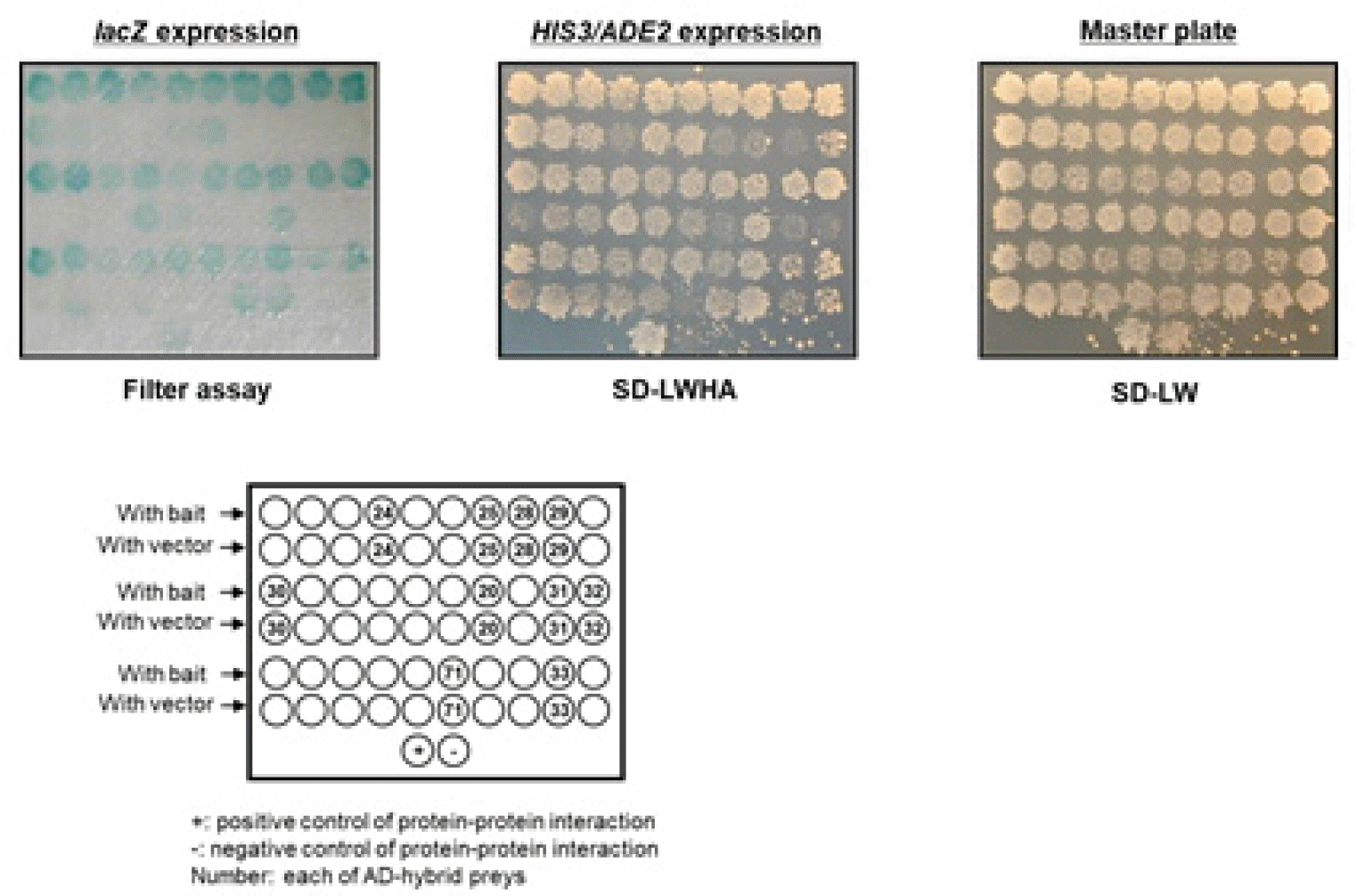 | Fig. 3Discovery of host factor binding to Nef through yeast two-hybrid screen.Nef bait (constructed to pGBKT-GAL4-AD) was transformed in AH109 strain which has HIS3, lacZ and ADE2 as reporter genes controlled under GAL4 promoter. The yeast transformants with the Nef bait were re-transformed with human thymus cDNA libraries (constructed to pACT2-GAL4-DB) and then grown on selection media [synthetic dropout (SD)-leucine, tryptophan, histidine and adenosine (SD-LWHA)] that supports growth of yeasts with interaction of bait and prey protein (middle panel). These yeast colonies grown on selection media also expressed lacZ reporter genes controlled under GAL4 promoter (right panel). The colonies without false positive were numbered and DB-cDNA from the colonies were identified as Nef-binding partner. AD: activation domain, DB: DNA binding domain. |
Go to : 
CLOSING REMARKS
Nef is a small protein, it plays an important role in various virulence-related mechanisms in HIV infected cells. However, the poorly understood the virulence mechanism of Nef. That is, no fundamental search has yet been conducted to identify a mechanism that commonly applies to MHC-1 down-regulation, CD4 down-regulation, and T cell signal activation by Nef, which thus limits our understanding of the overall virulence mechanism by Nef and further the utilization of treatment strategies by targeting these. For example, endocytic vesicle trafficking is involved in T cell activation signal by Nef, and down-regulation of CD4, SERIC5 (HIV-1 infection inhibitory protein), MHC-1, and many more membrane surface proteins by Nef is made through the lysosomal degradation pathway by Nef-induced endocytosis. In addition, many proteins are involved in Nef-induced virulence such as cell mobility inhibition and excessive secretory vesicle activity. To sum up, most of the Nef-induced virulence mechanisms discovered so far is related to the actin dynamics regulatory mechanism. Thus, the development of a strategy targeting Nef could primarily control Nef-induced virulence and provide the basic knowledge required for the development of novel drugs that induce asymptomatic infection, but only if we can discover the principal executor controlled by intrinsic Nef and determine how these interactions are involved in the induction of AIDS virulence.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download