INTRODUCTION
Cervical cancer is the 3rd most common cancer among women worldwide, with an estimated 569,847 new cases and 311,365 deaths in 2018. In Korea, about 3,600 women are diagnosed with cervical cancer and about 1,200 women die of this cancer each year. Currently, more than 190 HPV genotypes have been identified, the low-risk HPV genotypes including HPV-6/11 can cause infection that resolves spontaneously without specific symptoms, high-risk HPV genotypes including HPV-16/18 can induce malignant tumors (1). An HPV vaccine has been developed to prevent diseases caused by HPV infection, particularly cervical cancer. Three types of vaccines have been developed so far: 2-valent vaccine (including HPV-16/18, Cervarix, GlaxoSmithKline), 4-valent vaccine (including HPV-6/11/16/18, Gardasil, Merck & Co), and 9-valent vaccine (including HPV-6/11/16/18/31/33/45/52/58, Gardasil9, Merck & Co).
In Korea, bivalent and quardrivalent HPV vaccines were introduced as a NIP for girl aged 12 from June 2016. It has also been reported that HPV immunization has a preventive effect against HPV genotypes 16 and 18, which together cause 70% of cases of cervical cancer (2, 3). This article, aimed to review on the efficacy and immunogenicity of HPV vaccination in order to increase the HPV vaccination rate and verify its effectiveness in Korea.
Go to : 
BODY
Prophylactic HPV vaccines
Three prophylactic human papillomavirus (HPV) vaccines have high efficacy for prevention of infection and associated disease. The 2-valent vaccine was approved by the Australia in 2007 and by the KFDA in 2008, meanwhile, the 4-valent vaccine was approved by the US FDA in June 2006 and by the KFDA in June 2007. As for the 9-valent vaccine, it was approved in Korea as of January 2016 (Table 1) (5). The 9-valent HPV vaccine aims to prevent HPV-31/33/45/52/58 in addition to the genotypes targeted by the 4-valent vaccine. Both the 2- and 4-valent HPV vaccines showed high stability and efficacy in a 5-year long-term follow-up study. All vaccines are recommended 3 dose schedule for women aged 9-25 years prior to their first sexual contact.
Table 1.
Licensed prophylactic HPV vaccines (4)
Three vaccines contain synthetically manufactured virus like particles (VLPs) of the L1 epitope as immunogens. The expressed recombinant L1 capsids self-assembled in 72 pentamers that present an exterior surface very similar to the HPV virion and even in the absence of immune modulators (adjuvants). The Vaccines are classified according to the antigen expression system, antigen composition, and adjuvants included. Both Gardasil and Gardasil 9are produced in yeast and use aluminum hydroxyphosphate sulfate (AAHS) adjuvant, which has an increase the capacity to bind to L1 VLPs compared to aluminum salts. In contrast, Cervarix is produced in insect cells using baculovirus expression vector system, and adjuvant AS04 with contains aluminum hydroxide and monophosphoryl lipid A, a modified endotoxin and agonist of toll-like receptor. AS04 has been known to enhance innate immune responses, and may be responsible for differences in the overall immunogenicity (5). Other differences between vaccines include concentration of the L1 VLPs, and the ratio of antigen to adjuvant. Gardasil has two-fold higher concentration of HPV-16 L1 VLP compared to Cervarix, while their HPV-18 L1 VLP concentration is the same. In Gardasil9, the HPV-18 L1 VLP contains50% more than HPV-16 L1 VLP, and twice the level of adjuvant contained in Gardasil.
Introduction of the National Immunization Program (NIP) of HPV vaccine
The WHO emphasizes that HPV vaccines should be included in the NIP in each country, and at least 115 countries had included an HPV vaccine in their NIP (Table 2).
Table 2.
Countries including HPV vaccine in their National Immunization Programs by year of introduction (5)
In the USA, HPV 4-valent vaccine (Gardasil) was introduced to the NIP from 2006, after which the infection rate of HPV-6/11/16/18 decreased by 56% in 2007-2010 compared to 2003-2006 before the introduction of the NIP. In Australia, the 4-valent vaccine was introduced as a NIP for women aged 12-26 years from 2007. As a results, genital warts decreased by 89.7% in women under 21 years old compared to before vaccination and also decreased by 87.3% under 21 years old men who were not vaccinated because of herd immunity. In Denmark, the risk of CIN3 (cervical intraepithelial neoplasia 3) decreased by up to 80% after the introduction of 4-valent vaccine immunization compared to unvaccinated women.
Denmark introduced a 2-valent vaccine into its 2016 NIP, comprehensively investigated the efficacy and price of the Cervarix in July 2015. In the Netherlands, the 2-valent vaccine was evaluated to be highly cost-effective compared to the 4-valent vaccine, and thus was selected for the NIP. The UK started to use the 2-valent vaccine in 2008 for girls aged 12-13. According to the HPV prevalence of HPV infections surveyed from 2008 to 2014, HPV vaccination program remarkably reduced HPV-16/18 infection in young women. The UK placed greater emphasis on the prevention of genital warts, so switched to the 4-valent vaccine in 2012 based on the cervical cancer screening program. In Hungary, Belgium, and Singapore, a 2-valent vaccine was selected in consideration of its cost-effectiveness, and in South Africa, a bivalent vaccine with high immunogenicity was selected in consideration of the country's high HIV infection rates. In Korea, the 2-valent and 4-valent HPV vaccines have been included in the NIP since June 2016, which is administered to 12 years old girl with 2-dose schedule (3, 6).
In 2014, the 9-valent HPV vaccine, Gardasil 9, was approved by the FDA, 27 countries including the USA and Australia switched to or added Gardasil9 to their NIP. As Gardasil9 can provide a potentially wider range of protection, it is expected that 90% of the cases of cervical cancer in the world could be prevented, including about 87.7% in Asia, 91.7% in Africa, 92% in North America, 90.9% in Europe, 89.5% in South America, and 86.5% in Australia.
However, there is still a clear imbalance depending on the national income levels and efforts to prevent cervical cancer. Currently, 70% of cervical cancers occur in countries where HPV vaccination has not yet been introduced, and with lower-middle and low income countries (LMLIC) account for more than fifty percent of the burden of cervical cancer (40% and 16%, respectively). Most of the women in LMLIC are remain completely defenseless against cervical cancer due to lack of national HPV immunization program as well as effective screening program. Surprisingly, more than 93% of cervical cancer case occur in LMLICs that do not have a national HPV immunization program.
Global reaction to HPV vaccination over the past decade
According to the WHO guidelines, the protection and immunological correlation is not known for HPV vaccine efficacy, and diagnosis of cervical cancer is the most obvious clinical indicator, takes more than ten years to develop. Therefore, it is recommended that CIN2/3 and adenocarcinoma in situ (AIS) should be used as clinical indicator in studies on vaccines efficacy and long-term persistence. The representative clinical trials evaluating the efficacy of two vaccines include the Papilloma Trial (PATRICIA; HPV008) and the Costa Rica trial (CVT) of the 2-valent vaccine, and FUTURE (females united to unilaterally reduce endo/ectocervical diseases) I, II of the 4-valent vaccine). Several countries which introduced an HPV national immunization program earlier than Korea reported the HPV vaccine effects related to HPV infection and disease progression (Table 3).
Table 3.
Summary of Publications Reporting the Impact and Effectiveness of Human Papillomavirus Vaccination Programs in Countries (7)
Impact and Effectiveness of the bivalent human papillomavirus vaccine; Clinical trials
The 2-valent vaccine showed consistent preventive effects of 61%-75% in cervical cancer related lesions of CIN2 or worse, regardless of HPV genotypes, according to three independent clinical trials. These studies targeted women aged 15-25 (non-infected HPV) in four continents (PATRICIA study 64.9%, CVT 61.4%, Japan phase 2 trial 73.9%).
Impact and Effectiveness of the bivalent human papillomavirus vaccine; Clinical trials
The results of the FUTURE I, II clinical trials on the 4-valent vaccine showed a 98% preventive effect on CIN2/3 and AIS related to 4 genotypes (HPV-6/11/16/18), 3 years after the 1 dose vaccination, and the long-term follow-up study of about 2,000 women, aged 16-23, who participated in FUTURE II showed vaccine efficacy of 100% on HPV-16/18 for ten years, except CIN2/3. An almost 100% preventive effect was shown on HPV-6/11 related genital warts, along with an 83% preventive effect on all genital warts in individuals with no previous of HPV infection. However, there was no direct comparative clinical study on the efficacy of the 2-valent vaccine and the 4-valent vaccine, and the efficacy of the vaccines could vary depending on the number of participants in each 3-phase clinical trial. Therefore, it is not clear which vaccines are more effective in preventing cervical cancer (Fig. 1).
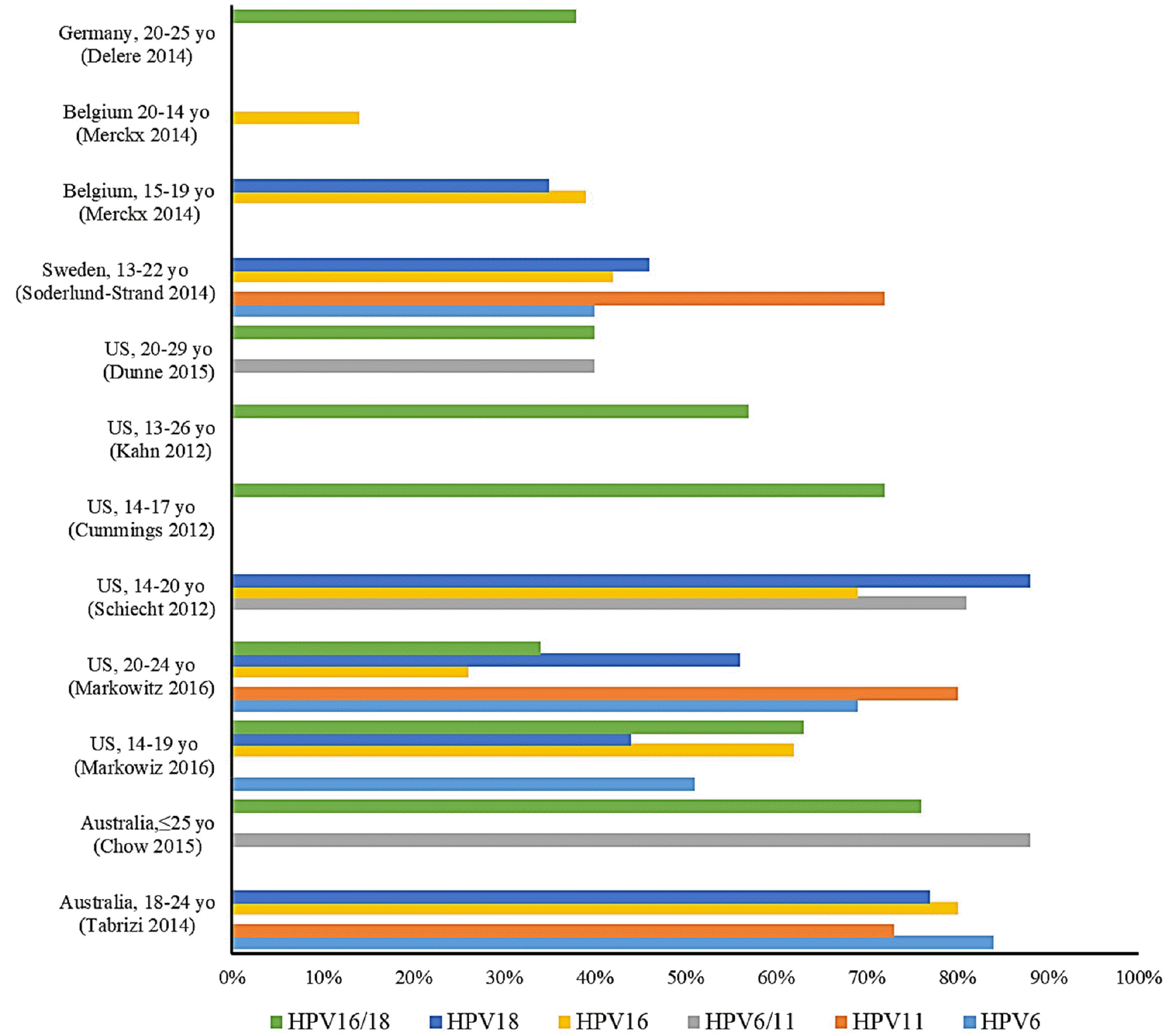 | Fig. 1Impact and effectiveness of quadrivalent human papillomavirus (HPV) vaccination on prevalence of vaccine genotypes (8). |
Impact and Effectiveness of the nonavalent human papillomavirus vaccine; Clinical trials
From September 26, 2007 to December 18, 2009, 14215 participants were recruited and randomly assigned to receive a vaccine of Gardisil 9 (n=7106) or Gardisil (n=7109). The incidence of HPV-31/33/45/52/58 related high-grade cervical, vulvar, and vaginal diseases was 0.5 cases per 10,000 person-years in the Gardasil9 and 19.0 cases per 10,000 person-years in the Gardasil group, representing 97.4% efficacy. There was no difference in the HPV-6/11/16/18 GMT (geometric mean titres) in the Gardasil9 verse the Gardasil group from month 1 to 3 years after vaccination. Furthermore, no clinically significant differences serious adverse events were found between the study groups. Thus, Gardasil9 could prevent the infections of cytological abnormalities, high-grade lesions, and cervical interventions, related to HPV-31/33/45/52/58. And Gardasil and Gardasil9 showed a similar immunogenicity profile with relation to HPV-6/11/16/18. Vaccine efficacy was sustained for up to 6 years.
Effectiveness of HPV vaccination in America
The United States conducted the NIP for females aged 11-12 years old in 2006. In 2010, vaccination coverage was 32%. According to the first study conducted to verify the efficacy of the 4-valent vaccine on 8,403 females aged 14-19 in the period 2003-2010 (comparative study before and after NIP introduction), the 4-valent vaccine type (HPV-6/11/16/18) was decreased from 11.5% in 2003-2006 (pre-NIP) to 5.1% in 2007-2010 (post-NIP) a decline of 56%. The vaccine effectiveness of at 1 dose was 82%. In 2008-2014, the prevalence of vaccine type, HPV-16/18 in cervical cancer was investigated in 10,206 women aged 18-39 with CIN2+. The number of cases of HPV-16/18 positive CIN2+ declined from 52.7% in 2008 to 44.1% in 2014, thus provides evidence of the vaccine impact in the United States. However, the efficacy of vaccines showed differences by age, disease grade, and race (9).
In Canada, the quadrivalent HPV vaccine was licensed for use in 2006, over the past decade (2006-2016), the prevalence of HPV-6 -1 -1618 was lower with4-valent HPV-vaccinated than unvaccinated individuals (1.5% vs. 11.0%, respectively), whereas non–vaccine genotypes were comparable across vaccination status. Risk of anogenital warts incidence was decreased by up to 45% in vaccinated cohorts and of cervical intraepithelial neoplasia 2+ incidence significantly reduced by up to 86% in the post vaccine cohorts. Based on these findings, HPV vaccines effectiveness appears to be resistant to this heterogeneity, and vaccine type associated reduction in HPV related infection and disease has been consistently observed throughout Canada (10).
Effectiveness of HPV vaccination in Europe
In France, 4-valent vaccine was introduced in 2007, and added the 2-valent vaccine in 2009. Since 2013, the vaccination has been recommended for 11-14 years old girls, with a catch-up from 15-19 years old. The vaccination rate was over 65% for women in the target age group and 4-valent vaccine was most often administered (93%). According to a study conducted to verify the effects of vaccination on women aged over 25 (a comparative study of 822 vaccinated cases verse 1,021 unvaccinated cases), the vaccine-related genotype (HPV-6/11/16/18) was 95.93% and that of the cross reactive genotype (HPV-31/33/45) was 38.37% compared to the unvaccinated group. Vaccine type HPV genotypes prevalence were significantly lower (0.61%) among confirmed-vaccinated women than unvaccinated women (1.76%). Thus, High vaccine coverage could result in dramatic reduction in prevalence of infection with genotypes responsible for 70% of cervical cancers and 56% of precancerous lesions and translate into a decline in cervical disease. The impact of HPV vaccination depends on vaccine coverage (11).
In 2008, the UK began a HPV immunization program in adolescent girls (aged 11-12 years, with catch-up to 17 years). The prevalent of the HPV-16/18 genotype, which were targeted in the 2-valent vaccine, decreased from 8.2% to 1.6% in girls aged 16-18 and from 14.0% to 1.6% in women aged 19-21 years in 2010/2011-2016. Also, the prevalence of HPV-31/33/45 types was decreased in16-18 years old, from 6.5% to 0.6%; in 19-21 years old, from 8.6% to 2.6%). In conclusion, the 2-valent vaccine demonstrated a preventive effect including 82.0% of HPV-16/18 and 48.7% of HPV-31/33/45. Also, the incidence of penile cancer was decreased to 3,433 cases and that of head cancer and laryngeal cancer to 21,395 cases in the same period. However, the prevalence of other high-risk HPV types has not changed. In 2012, the program switched to the 4-valent vaccine additionally protects against HPV types HPV-6/11, which are responsible for over 90% of case of genital warts. In oropharyngeal cancer, the prevalence of the HPV-16 was a significantly lower in vaccinated verses unvaccinated females (0.5% vs. 5.6%); in contrast, the prevalence of non-vaccine HPV types was similar in vaccinated and unvaccinated women (19% vs. 20%, P=76). The prevalence of HPV-16 in unvaccinated males was similar to vaccinated females (0% vs. 0.5%), and lower than unvaccinated females (0% vs. 5.6%). These results showed a potential herd immunity in males of a similar age due to the vaccination in females. Thus, Public Health England (PHE) predicted that about 100,000 cases of cancer could be prevented by 2058 (12), (13).
Effectiveness of HPV vaccination in Oceania
In Australia, National Human Papilloma Virus Vaccination Program for prevent HPV infection and other related diseases using the 4-valent HPV vaccine has been implemented in 2007, initially for girls aged 12-26 only and expanded to boys in 2013, with vaccination rates among the highest observed in the world. After the introduction of the vaccination program, prevalence of vaccine type (HPV-6/11 or HPV-6/11/16/18) with uterine cancer decreased by over 77% in women aged 18-24 and by 34% in women aged 20-24. This means that the risk of cervical cancer is now reduced. In general, the incidence of high-risk cervical diseases decreased by more than 50% in vaccinated women.
The genital warts incidence and HPV prevalence among heterosexual men of similar age was declined before introduction of the male vaccination program, indicating a substantial herd effect. Vaccination with the 4-valent vaccine greatly influenced the incidence of HPV-related diseases in Australia. Also, after the introduction of the 9-valent vaccine the incidence of all genotypes decreased from 58.1% to 36.2% (2005-2014, Australian indigenous women). In the case of high-risk group genotypes, they decreased from 42.6% to 17.7%. Particularly, the HPV-16/18 type decreased from 32.9% to 17%. The 9-valent related genotype decreased from 34.8% to 10.6%, and the 4-valent related genotype decreased from 23.9% to 1.4%. A switch to 9vHPV could further reduce the HPV-associated cancer burden. Australia predicts that the transition to the 9-valent vaccine will prevent HPV-related cancer burden by 90% of cervical and 96% of anal cancer (9), (14).
Effectiveness of HPV vaccination in Asia
In April 2010, Japan introduced the HPV vaccine program in women aged 11-45 in Akita, and expanded it to include girls aged 12-16 as part of the National Immunization Program in November 2010. The prevalence of HPV-16/18 and high-risk HPV and the incidence of HPV-related cervical disease were investigated in women aged 18-49 years who visited hospitals during the period 2008-2017 (a comparative study pre vaccine era verse vaccine era). In women aged 18-24, the prevalence of HPV-16/18 and high-risk HPV was decreased from 36.7% and 69.4%, in the pre-vaccine era to 5.8% and 50%, respectively, in the vaccine era. Among women with CIN2 and CIN2+, the prevalence of HPV-16/18 decreased from 30.0% to 2.7% and from 81.8% to 36.4%, respectively. The HPV-16/18 positive rate was significantly decreased (68.2%) in the group aged 18-27 with a high vaccination rate, but the prevalence of HPV in the other age group with lower vaccine coverage (25-49 years) did not significantly differ between eras (15).
Current status of HPV infection and vaccination in Korea
In 2016, HPV vaccine, the 2-valent and the 4-valent vaccines, was introduced in the National Immunization Program in Korea as a 2 dose schedule for girls aged 12, and the vaccination coverage has increased in targeted aged. The prevalence of HPV infection in Korea is estimated to be about 10%-15%, at young women (aged 19-20 years old) and in 38.8% of women with sexual experience were infected with HPV.
According to a Meta-analysis of HPV infection rate in Korea between 1995 and 2007, the HPV infection rate was 20.4% and the high-risk HPV infection rate was 16.7% in women with a normal cervix. The HPV infection rate of atypical squamous cells of undetermined significance (ASCUS/CIN1) was 63.2% and the high-risk HPV infection rate was 56.3%. The HPV infection rate of in CIN2/3/CIS patients was 85.6% and the high-risk HPV infection rate was 83.7%. Among cervical cancer patients, the HPV infection rate was 88.3% and the high-risk HPV infection rate was 84.6%, the highest infection rate among HPV types was HPV-16 followed by HPV58 and 18.
When compared to the results of studies conducted in East Asia, the results for the five most frequent types were similar for the whole of Asia. The analysis of the HPV infection rate of cervical precancerous lesions and cancers by genotype showed that the most frequently found genotypes in domestic cervical diseases are HPV-16 and HPV-18, which were detected in 65.1% of all cervical cancer patients, and the 5 types of major high-risk HPV types (HPV-16/18/58/33/52) were detected in cervical cancer. In 2009, HPV-16/18 prevalence was found to be 8.7% in 1,094 Korean females aged 9-59; to be highest at 13.4% in women aged 25-29 years, decreasing to 7.6% in women aged 30-39 years, and then increasing again to 10.9% in women aged 40-49 years.
The KCDC recommends the vaccination of girls aged 11-12 (before first sexual contact), which is the optimal age for vaccination with the 2-valent and 4-valent vaccines. In a survey of HPV vaccination in Asia, the rate of vaccination in teen girls and adult women in 2008 was less than 4% vaccination coverage was increased at around 80% after NIP in targeted age in 2019. According to the general public's perception and vaccination rate after the inclusion of the HPV vaccine in the NIP, the importance of HPV infection increased from 13.3% in 2007 to 35.8% in 2016, and the reliability of the vaccine's effect increased from 8.6% in 2007 to 36.9% in 2016. In addition, perception of the adverse side effects of the vaccine decreased from 55% to 25.8% (2), (3).
Current research and future direction in KNIH
According to “Status of HPV vaccination among HPV-infected women aged 20–60 years with abnormal cervical cytology in South Korea: a multicenter, retrospective study” (1) the vaccination rate in adult women aged 20-60 was about 25.8% in 2010-2016, and the ratio of vaccination with the 2-valent vaccine and the 4-valent vaccine was about 2:1. In HPV-positive women (aged 20-60 years) with atypical squamous or atypical squamous intraepithelial lesion (LSIL), the prevalence of vaccine-related genotypes (HPV-16/18) decreased in the vaccinated group (11% vs. 17%).
We found that for at least 12 months after HPV vaccination, the prevalence of HPV-16/18 in adult women diagnosed with LSIL was significantly lower (Table 4). It is expected that our results will provide the preliminary information for studies to verify the effects of HPV vaccines after NIP.
Table 4.
Prevalence of HPV-16/18 in HPV-positive women by vaccination (1)
Go to : 
CONCLUSION
To verify the effects of vaccines, there are several limitations including vaccine type specificity, age, number of vaccinations, sexual experience at vaccination, presence of HPV infection at vaccination, and potential communication problems. As the design, implementation, and interpretation of a study at verifying the effects of a vaccine is a difficult and complex, sufficient clinical epidemiological data should be collected.
In Korea, cervical screening coverage was higher than 67% in women aged 20-74. Eventually, it is estimated that data for verifying the effects of vaccines will be provided if the HPV DNA test is performed in combination with the cervical screening. As herd immunity has been reported in men of similar ages with vaccinated women in the USA and Australia, it is a top priority to increase the vaccination rate, and it is also necessary to review the need for separate preventive measures, such as enhanced sex education, in addition to vaccination.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download