INTRODUCTION
Over 300 million people, about 4% of the world’s population, are chronically infected with hepatitis B virus (HBV), and a significant number of these patients also suffer from liver diseases such as cirrhosis and liver cancer. In East Asia (especially Taiwan, Japan, Korea and China), HBV is prevalent, with the number of infected people in Korea estimated to be over 2 million, accounting for 60~70% of chronic liver diseases (1, 2). In over 60% of the cases in Korea the infection route for chronic hepatitis B is vertical transmission from mother to infant at childbirth. Treatments for hepatitis B are interferon alpha injections including Peg-interferon and orally-administered nucleo(t)side analogs. Interferon agents have a fixed administration period and are expected to improve the biochemical and histological findings due to their virus-suppressing effect and immune- modulating action, but their use has declined due to the low treatment reaction and adverse side effects. As for nucleo(t)side analogs, they only inhibit DNA synthesis, and if their administration is stopped a viral breakthrough can develop, making long-term administration necessary, ultimately followed by the development of resistance. Recently, Tenofovir (TDF) has been developed and drug-resistant mutations have decreased significantly, but the problem of resistance by long-term drug use still remains, along with the drug safety problem, due to the treatment characteristics of chronic hepatitis B (3). As such, this paper examines the recent trend in the development of hepatitis B treatment agents.
Go to : 
BODY
Characteristics and life cycle of HBV
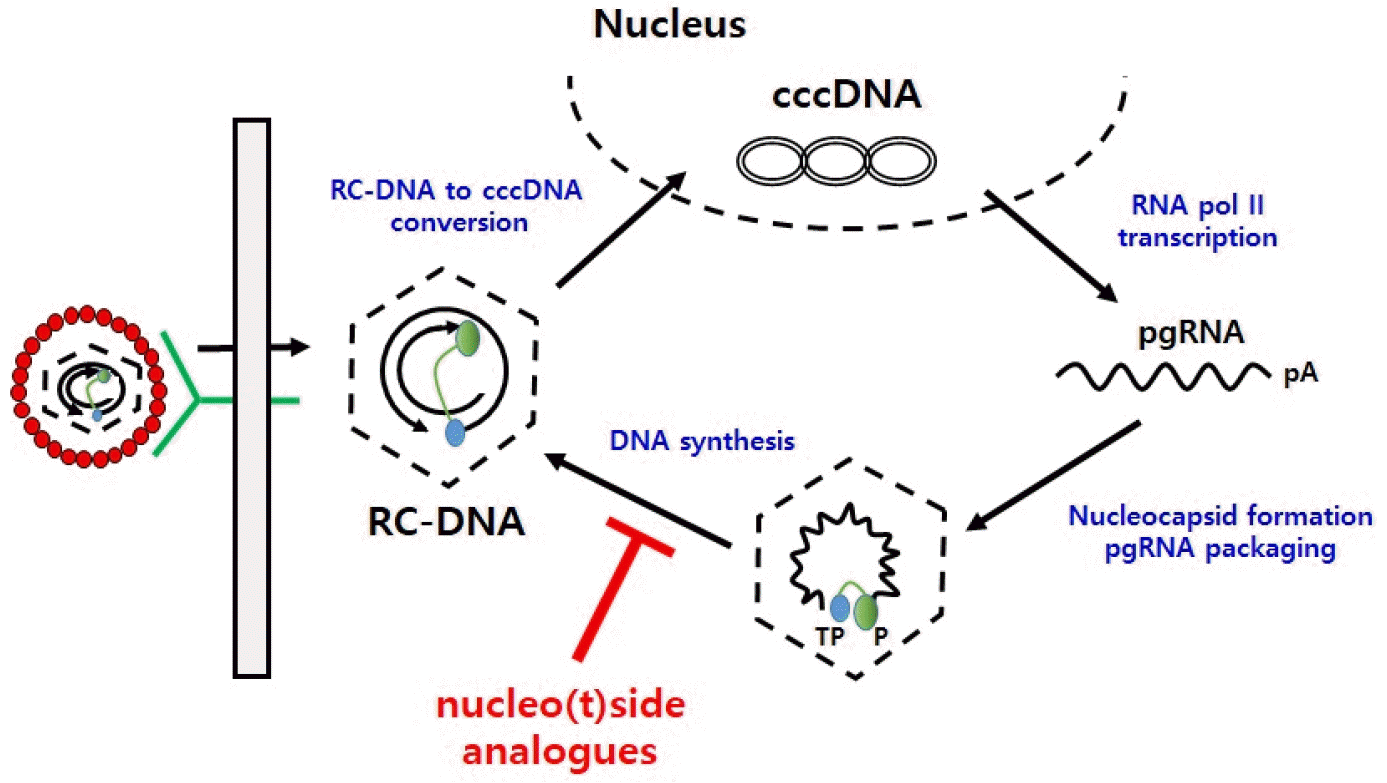
HBV is a 3.2kbp partially double-strand DNA virus which exists as a complete form of Dane particles with infectivity surrounded by capsid and envelop. The DNA is enclosed by a capsid made of core proteins, which are in turn enclosed by surface proteins. HBV characteristically infects only liver cells (hepatotropic) and causes persistent infection without the degeneration of infected cells (non-cytopathic). When the HBV virion infects the liver cells, surface proteins are released. Then the viral gene inside the capsid enters into the nucleus and the partially double-stranded HBV DNA transforms into a circular type of cccDNA. The viral RNA produced from the cccDNA produces core and surface proteins and polymerase, and encapsidation progresses in the cytoplasm with pre-genomic RNA (pgRNA), which can be converted to the original viral DNA. After the conversion from pgRNA to DNA, the HBV virion goes through the budding process, after which it can infect or re-infect the surrounding liver cells, causing persistent proliferation (4).
pgRNA is transcribed to the DNA within the capsid, and at this time nucleoside analogs cut in to the newly synthesized DNA strand and terminate the synthesis. HBV polymerase consists of four domains, and various drugs have been developed to target the reverse transcriptase (RT) domain where DNA is synthesized from RNA; and the structural changes of the active site due to mutations of the RT domain are the cause of drug resistance (Fig. 1).
HBV treatment agents
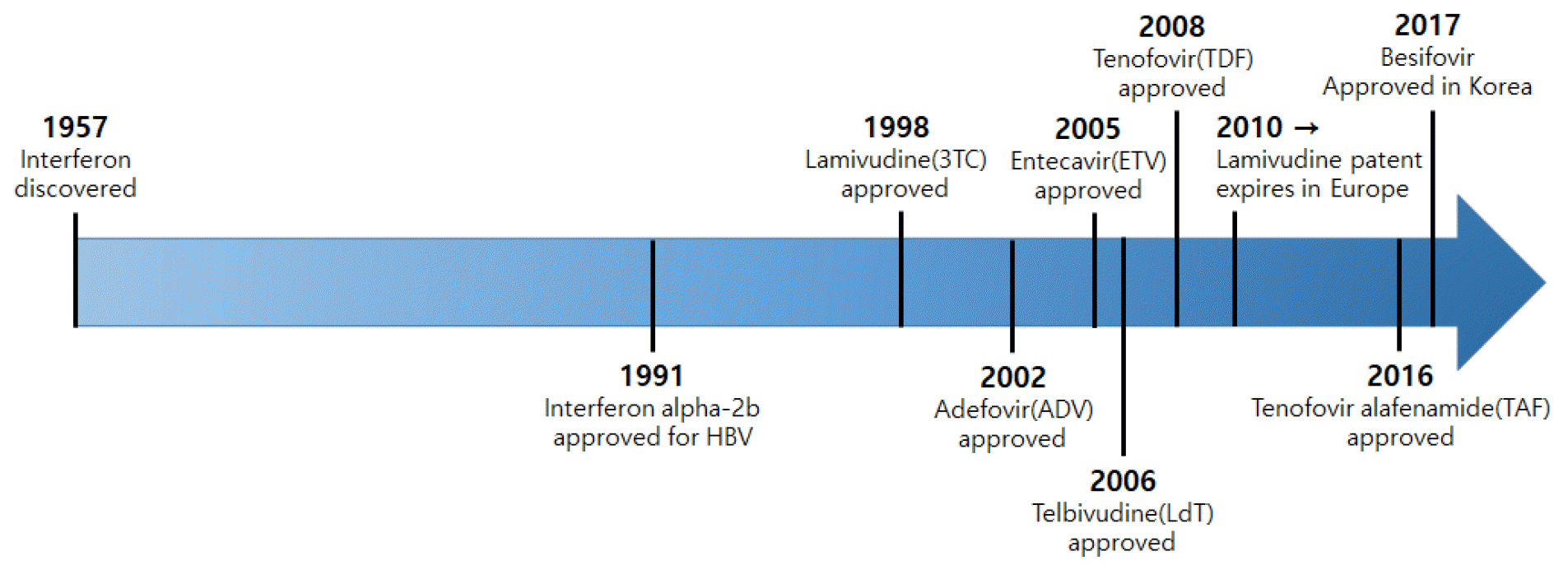
The full-scale treatment of chronic hepatitis B began in the early 1990s when interferon was first used (5). Then, after the first FDA approval of Lamivudine (LMV) in 1998, nucleo(t)side analogs such as Adefovir (ADV), Entecavir (ETV), Telbivudine (LdT), Clevudine (CLV), and Tenofovir (TDF) came to be used worldwide (Table 1) (6). Despite the various advantages of interferon (IFN-alpha), it is inconvenient to use as an injection and is of limited use for patients with decompensated cirrhosis; thus, orally administered antiviral agents are currently the mainstream treatment for chronic hepatitis B. As tenofovir alafenamide fumarate (TAF) and besifovir dipivoxil maleate (Besifovir, BSV) were approved as a treatment medication for adults with chronic hepatitis B in South Korea in 2017, a total of eight antiviral agents can now be used (Fig. 2).
Table 1.
HBV treatment drugs available for chronic Hepatitis B virus (HBV) infection (Modified from Tang LSY et al. 2018)
Lamivudine (LMV, 3TC)
LMV was originally developed for AIDS treatment as an inhibitor for HIV reverse transcriptase, and was also approved as a hepatitis B treatment drug, i.e. a 1st-generation nucleotide analogue, by the FDA in 1998 because it effectively inhibits HBV reverse transcriptase (7). LMV is successively phosphorylated to LMV triphosphate by intracellular kinase, and shortly after the diphosphate groups are eliminated, LMV 5’-monophosphate is incorporated into the newly produced viral DNA by HBV polymerase. Because it is a nucleoside analog without a 3’-OH group for chain polymerization, it induces the termination of polymerization synthesis and inhibits viral replication. Although the action mechanism of most antiviral agents is similar to this, drug-resistant mutation within five years is as high as 70%.
Adefovir (ADV)
ADV, an adenosine monophosphate analogue, is phosphorylated by intracellular kinase and activated to ADV diphosphate, whereupon it competitively inhibits the reaction deoxyadenosine triphosphate, a substrate of HBV DNA polymerase. ADV diphosphate is incorporated into newly produced viral DNA, and has an antiviral effect caused by a similar action mechanism to that of LMV, but the recommended dosage is 1/10 (10 mg/day) that of LMV, and its rate of drug-resistant mutation within 5 years has been improved by 20-29% (6). Although ADV had an important role for LMV-resistant patients, it is no longer recommended as a first-line therapy.
Entecavir (ETV)
ETV, a guanosine nucleoside analogue approved by the US FDA in March 2005, is known to show the more enhanced drug effect (0.5~1 mg/day) compared to other existing drugs, is less likely to cause an adverse reaction, and has only a 1.2% incidence of drug-resistant mutation within five years (8). Because of this excellent resistance rate, ETV is recommended as a first-line therapy for chronic HBV (9).
Telbivudine (LdT)
LdT, a thymidine nucleoside analogue, is an unmodified L-isomer of thymidine, a naturally occurring nucleoside. Thus, the phosphorylation reaction to the active form LdT triphosphate occurs easily. However, LdT has not been used for a first-line therapy because of the higher frequency of resistance in early time during administration similar to LMV (9).
Clevudine (CLV)
CLV approved in 2006 in South Korea, a pyrimidine analogue (30 mg/day), is known to not only inhibit DNA-dependent DNA activity for HBV polymerase but also to demonstrate an antiviral effect by interrupting reverse transcription and priming. CLV is about 10-fold more potent than LMV against HBV in cell culture, and HBV DNA level was reduced by 2.5 to 3 log10 copies/mL for 4 weeks trial with 10 to 200 mg/day (10). CLV is being marketed in Korea and Philippines named as Lenovir and Revovir, respectively.
Tenofovir (TDF)
Tenofovir disoproxil fumarate, a prodrug of Tenofovir (TDF), is an oral antiviral agent approved for HIV and HBV treatments (11). TDF is successively phosphorylated by intracellular kinase and activated to tenofovir diphosphate, and then competes with endogenous nucleotide deoxyadenosine 5'-triphosphate (dATP) for incorporation into the newly replicated HBV DNA by HBV polymerase. Incorporated TDF, instead of endogenous nucleotide, is a nucleotide analogue without a 3’-OH group, which is required for the elongation of DNA base chains, and thus it induces the termination of polymerization synthesis and inhibits viral replication (12). This action mechanism is very similar to that of another nucleotide analogue, ADV, but the antiviral efficacy of TDF is much stronger than that of ADV because the latter is used in a limited dosage (only 10 mg) so as to reduce the development of nephrotoxicity, whereas TDF is used in a far higher dose of 300 mg (13). Also, it is assumed that the higher binding affinity of TDF to HBV polymerase, compared to ADV, is related to its strong efficacy (14). Drug-resistant mutation was not reported initially, but mutations have been observed more recently (6, 15).
Besifovir (LB80380)
Besifovir is an acyclic nucleotide phosphonate similar to Adefovir or Tenofovir. It is a nucleotide analogue and a prodrug of guanosine triphosphate nucleotide analogue LB80317. Besifovir is absorbed into the intestine and deacetylated to the intermediate metabolite LB80331 by esterase in the intestine and the liver, and then oxidized to the active metabolite LB80317 by oxidase (aldehyde oxidase or xanthine oxidase). In the liver cells it is phosphorylated to diphosphate and triphosphate forms, after which it competes with dGTP to bind with HBV DNA polymerase, and thus blocks the action of polymerase and inhibits viral proliferation. After the phase 2 clinical trial conducted by LG Life Sciences, Korea and the phase 3 clinical trial by Ildong Pharmaceutical, Korea, it was released on the market in 2017 as a domestic novel drug, with a similar efficacy to that of TDF, but without the latter’s adverse side effect of reduced bone density (16).
Tenofovir alafenamide fumarate (TAF, GS7340)
TAF, a prodrug of Tenofovir used in the body and a nucleotide analogue like TDF, is an oral antiviral agent that inhibits reverse transcription from pre-genomic RNA to DNA. Prodrugs are being developed to reduce its adverse effects on the functions of the kidneys by increasing bioactivity and enhancing the antiviral action compared to the strong antiviral agent TDF. The most representative drug is TAF. TAF is converted to tenofovir-alanine conjugate (TAF-Ala) in the body and then converted again to TDF and phosphorylated to active metabolite tenofovir diphosphate (TAF-DP) for drug action. Recently, the results of a phase 3 clinical trials for comparison with TDF in HIV patients showed similar antiviral effects, but TAF showed significantly better responses compared with the disadvantages of TDF, such as increased serum creatine, proteinuria, and reduced bone density. In addition, studies on CMX157, a hexadecyloxypropyl conjugate of TDF, and on AGX-1009 of another structure, are currently in progress.
HBV drug resistance
For the effective treatment of viruses, drugs are administered by monotherapy or combination therapy. Methods with the least incidence of resistant viruses and a quick treatment for HBV have been widely studied and are currently being applied in clinical situations.
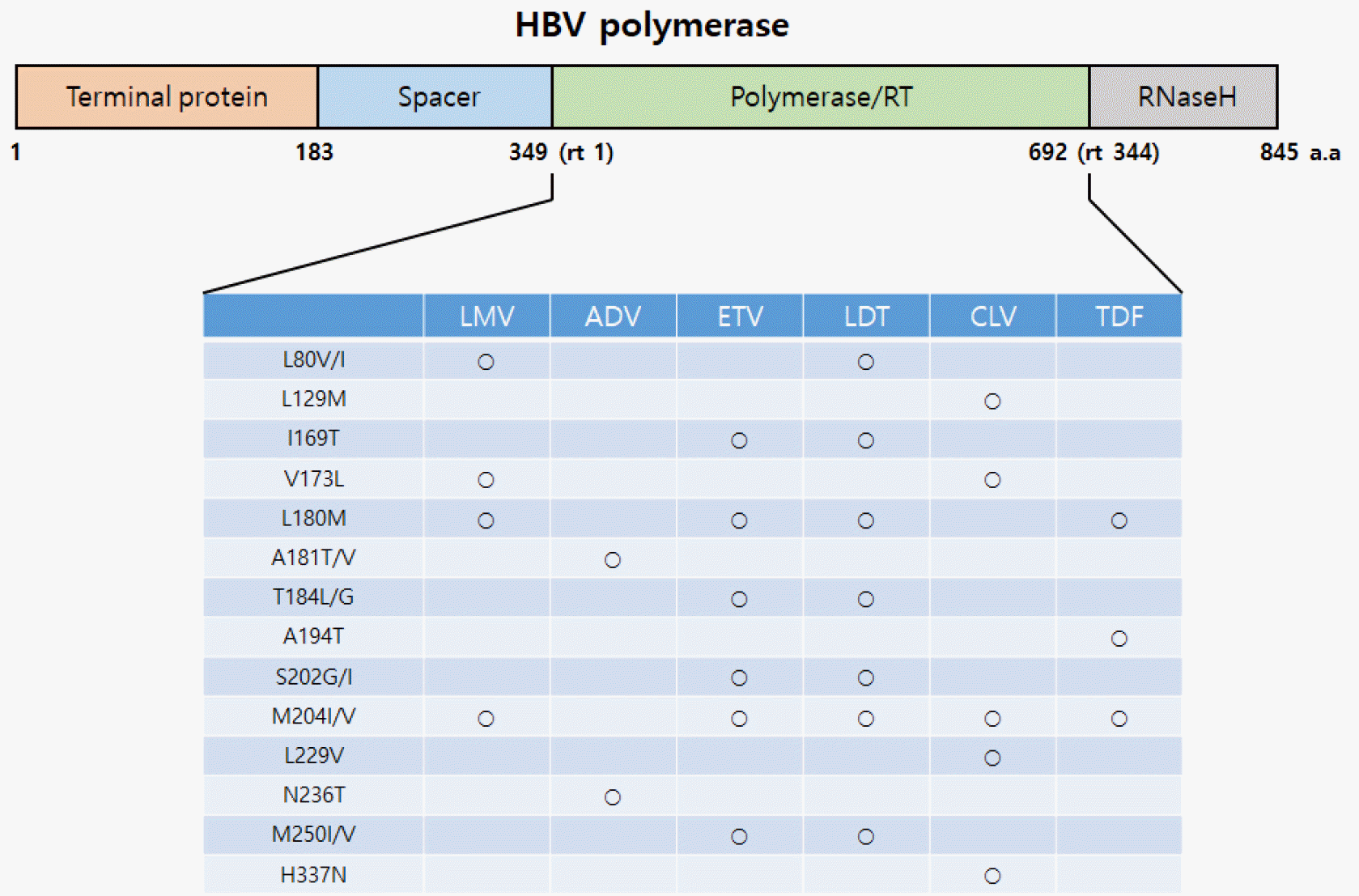
The long-term administration (i.e. more than one year) of a medication for chronic HBV infection leads to the development of drug-resistant mutation viruses in many cases. This is because there is an active site in the center of HBV polymerase where DNA synthesis occurs; if mutations of the amino acids inherent in each drug occur near this site, drugs cannot incorporate into the site due to steric hindrance, whereupon resistance develops. Ultimately, it is most important to select the optimal antiviral agent for treatment by continuously monitoring each drug for the development of a mutation after its administration, because each drug displays different resistance patterns and mutations during long-term administration. The characteristics of resistance for each antiviral agent studied up to the present time are as follows (Fig. 3).
Lamivudine resistance
The incidence of resistant viruses during LMV treatment has increased by 14~32% each year, and the drug shows high resistance of about 80% after 48 months (17). The primary resistance mutation for LMV is the YMDD motif of the C domain of HBV polymerase. It is an rtM204I/V mutation in which YMDD is changed to YIDD or YVDD. In addition, mutations L80V/I, V173L, and L180M have been observed found in the RT areas.
Adefovir resistance
The incidence of ADV resistance is known to be lower than that of LMV. In HBeAg-negative patients, resistance develops in about 2% of patients after two years of ADV monotherapy, but resistance has been observed in almost 30% of patients after five years of monotherapy. HBV RT domains in rtA181T/V and rtN236T are known ADV resistance mutations (18). In an in vitro drug susceptibility assay, rtN236T mutation did not affect the susceptibility for LMV, LdT, and ETV, but rtA181T mutation reduced the susceptibility for LMV (< 10-fold), ADV (< 2-8 fold), and TDF (< 2-3 fold).
Entecavir resistance
ETV shows the strongest antiviral effect among the drugs currently being used. No resistance was observed initially, and it was observed in less than 1% of patients during the two-year period (19). Also, it is effective in LMV-resistant rtL180M and rtM204V (20). Until now, ETV resistant mutations have been found in the B domain (rtI169T, rtL180M, rtT184L), C domain (rtM204I/V, rtS202G/I), and E domain (rtM250I/V). However, Entecavir resistance does not cause cross resistance in ADV or TDF.
Telbivudine resistance
The primary resistance mutation of LdT is known to be rtM204I/V, often accompanied by rtL80I/V and rtL180M. The frequency of resistance after two years of therapy is lower than for LMV but higher than for ADV or ETV. In an in-vitro drug susceptibility experiment, rtM204I, rtL180M/rtM204V, rtI169T/rtM250V, and rtT184G/rtS202I were shown to be mutations for resistance (21). Because LdT is an L-nucleoside analogue, LdT resistance does not cause cross resistance with ADV, TDF or ETV, and thus is used as an alternative treatment for resistance to those drugs (22).
Clevudine resistance
The primary resistance mutation of CLV includes rtM204I and the recently discovered rtL229V as a compensatory mutation for this. Also, the quadruple mutation of rtL129M+rtV173L+rtM204I+rtH337N, which exhibits very strong resistance, to both CLV and LMV (23), has been discovered
Tenofovir resistance
TDF is known to be the drug with the least resistance. In TDF treatment of patients with both HBV and HIV infection, the rtA194T mutation was found along with the rtL180M + rtM204V mutation, but in an in vitro experiment using a cell line, the rtA194T mutation showed partial resistance to TDF regardless of the rtL180M+rtM204V mutation (about 5~7 times) (24). A treatment-naïve patient showed the TDF-resistance at rtY9H, rtL91L, rtS106C, rtH126Y, rtD134E, rtQ267L, rtI269L, rtA317S, rtK333Q and rtN337H, but the clinical significance remains unclear (25).
Besifovir resistance
In a multi-centered phase 2b clinical trial conducted to compare the treatment effect of BSV (90 mg or 150 mg) and ETV 0.5 mg, a viral breakthrough was observed at 16 weeks only in 1 patient with lower drug compliance among the 31 patients to whom BSV 90 mg was administered, but no viral mutation related to BSV resistance was observed (26).
Tenofovir alafenamide fumarate resistance
KNIH (Korea National Research Institute of Health)
Research background and goals
Nucleo(t)side analogs such as LMV, ADV, ETV, and TDF are currently being used to inhibit HBV replication for the treatment of chronic hepatitis B, but long-term administration of these drugs leads to the development of drug resistance, making a complete cure impossible; thus it is urgent to develop drugs that target various parts of the life cycle of HBV. At the KNIH, studies are under way to investigate the cccDNA regulation mechanism for complete cure of HBV. To identify the potential antiviral effects by discovering the intracellular host factors, candidate antiviral molecules are tested for the inhibition of cccDNA formation.
Research content
Host factors that regulate the MAPK (mitogen-activated protein kinase) signaling system related to HBV replication were discovered and their influences on HBV life cycle were studied. For this, a hepatoma cell line was manufactured in which the discovered host factors can be stably expressed. Then, it was observed that the secretion of HBV antigens (HBeAg, HBsAg), DNA replication, RNA, and protein expression decreased. Also, the inhibition of mRNA and protein expression of the transcription factor HNF4α (hepatocyte nuclear factor 4 alpha) that binds to the HBV enhancer was observed. In conclusion, it was identified that the discovered host factors inhibit the activity of the HBV enhancer and suppress HBV transcription. As such, the chemical compounds related to this have been selected and their HBV inhibition effect is now being analyzed. It is considered that the results of this study will provide the scientific basis for the development of a novel treatment for hepatitis B (drug repositioning) without resistance and which targets the various life cycles of HBV.
Go to : 
CONCLUSION
Currently used antiviral drugs for HBV are viral polymerase inhibitors. LMV has mainly been used as an initial treatment drug for chronic hepatitis B, while ADV has been used as an alternative for LMV resistance. As shown in Fig. 3, the long-term use of HBV treatment drugs can induce drug-resistant mutation within the active site of the reverse transcriptase domain of HBV. Compared to the antiviral agents such as ADV, ETV, and LdT, TDF has been used because these are strong agents with less resistance even during long-term administration, but further studies are necessary whether recently reported mutations are clinically significant.
Currently available drugs are unable to achieve the complete eradiation of the cccDNA of chronic hepatitis B, resulting to an inactive carrier state with suppressed viral replication and continuous surveillance for liver cancer. Therefore, it is essential to develop antiviral agents with a totally different action mechanism targeting the HBV life cycle, for example, inhibiting the HBV entry to hepatocytes, the HBx interaction, HBV core assembly, and HBV entry to the hepatocytes (29). Also, new immunomodulatory therapies targeting HBV such as Toll-like receptor agonists have been developing for overcoming the host tolerance.
It is no doubt that nucleo(t)side is the first-line therapy, but occurring the mutations during long-term administration are limitation for complete cure of HBV. Further studies on a combination therapy using a nucleo(t)side analogue and novel targeting antiviral agents to HBV life cycle and immune response should be conducted for cure the HBV without the adverse effects.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download