1. Hachamovitch R, Berman DS, Kiat H, Cohen I, Friedman JD, Shaw LJ. Value of stress myocardial perfusion single photon emission computed tomography in patients with normal resting electrocardiograms: an evaluation of incremental prognostic value and cost-effectiveness. Circulation. 2002; 105:823–829. PMID:
11854122.

2. Xaplanteris P, Fournier S, Pijls NH, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018; 379:250–259. PMID:
29785878.

3. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018; 39:3759. PMID:
30403801.
4. Lee JM, Jung JH, Hwang D, et al. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol. 2016; 67:1158–1169. PMID:
26965536.

5. Lee JM, Choi KH, Hwang D, et al. Prognostic implication of thermodilution coronary flow reserve in patients undergoing fractional flow reserve measurement. JACC Cardiovasc Interv. 2018; 11:1423–1433. PMID:
30093048.
6. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020; 41:407–477. PMID:
31504439.
7. Hamaya R, Yonetsu T, Kanaji Y, et al. Diagnostic and prognostic efficacy of coronary flow capacity obtained using pressure-temperature sensor-tipped wire-derived physiological indices. JACC Cardiovasc Interv. 2018; 11:728–737. PMID:
29605243.

8. Echavarria-Pinto M, Escaned J, Macías E, et al. Disturbed coronary hemodynamics in vessels with intermediate stenoses evaluated with fractional flow reserve: a combined analysis of epicardial and microcirculatory involvement in ischemic heart disease. Circulation. 2013; 128:2557–2566. PMID:
24141255.
9. Mejía-Rentería H, Lee JM, Lauri F, et al. Influence of microcirculatory dysfunction on angiography-based functional assessment of coronary stenoses. JACC Cardiovasc Interv. 2018; 11:741–753. PMID:
29673505.

10. Johnson NP, Kirkeeide RL, Gould KL. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging. 2012; 5:193–202. PMID:
22340827.

11. De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014; 371:1208–1217. PMID:
25176289.

12. Lee JM, Layland J, Jung JH, et al. Integrated physiologic assessment of ischemic heart disease in real-world practice using index of microcirculatory resistance and fractional flow reserve: insights from the International Index of Microcirculatory Resistance Registry. Circ Cardiovasc Interv. 2015; 8:e002857. PMID:
26499500.

13. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011; 123:2736–2747. PMID:
21670242.
14. Authors/Task Force members. Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014; 35:2541–2619. PMID:
25173339.
15. Ahn JM, Park DW, Shin ES, et al. Fractional flow reserve and cardiac events in coronary artery disease: data from a prospective IRIS-FFR Registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation. 2017; 135:2241–2251. PMID:
28356440.
16. Lee JM, Koo BK, Shin ES, et al. Clinical implications of three-vessel fractional flow reserve measurement in patients with coronary artery disease. Eur Heart J. 2018; 39:945–951. PMID:
29020260.

17. Li J, Elrashidi MY, Flammer AJ, et al. Long-term outcomes of fractional flow reserve-guided vs. angiography-guided percutaneous coronary intervention in contemporary practice. Eur Heart J. 2013; 34:1375–1383. PMID:
23344979.

18. van de Hoef TP, van Lavieren MA, Damman P, et al. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014; 7:301–311. PMID:
24782198.

19. van de Hoef TP, Bax M, Damman P, et al. Impaired coronary autoregulation is associated with long-term fatal events in patients with stable coronary artery disease. Circ Cardiovasc Interv. 2013; 6:329–335. PMID:
23899871.

20. Meuwissen M, Chamuleau SA, Siebes M, et al. The prognostic value of combined intracoronary pressure and blood flow velocity measurements after deferral of percutaneous coronary intervention. Catheter Cardiovasc Interv. 2008; 71:291–297. PMID:
18288725.

21. Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012; 5:430–440. PMID:
22498334.

22. Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006; 113:2054–2061. PMID:
16636168.
23. de Bruyne B, Bartunek J, Sys SU, Pijls NH, Heyndrickx GR, Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996; 94:1842–1849. PMID:
8873658.
24. Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014; 129:2518–2527. PMID:
24787469.

25. Taqueti VR, Ridker PM. Inflammation, coronary flow reserve, and microvascular dysfunction: moving beyond cardiac syndrome X. JACC Cardiovasc Imaging. 2013; 6:668–671. PMID:
23764095.
26. Camici PG, Olivotto I, Rimoldi OE. The coronary circulation and blood flow in left ventricular hypertrophy. J Mol Cell Cardiol. 2012; 52:857–864. PMID:
21924273.

27. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014; 35:1101–1111. PMID:
24366916.

28. Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012; 33:829–837. PMID:
21890489.
29. Dhawan SS, Corban MT, Nanjundappa RA, et al. Coronary microvascular dysfunction is associated with higher frequency of thin-cap fibroatheroma. Atherosclerosis. 2012; 223:384–388. PMID:
22766333.

30. Usui E, Yonetsu T, Kanaji Y, et al. Optical coherence tomography-defined plaque vulnerability in relation to functional stenosis severity and microvascular dysfunction. JACC Cardiovasc Interv. 2018; 11:2058–2068. PMID:
30336810.

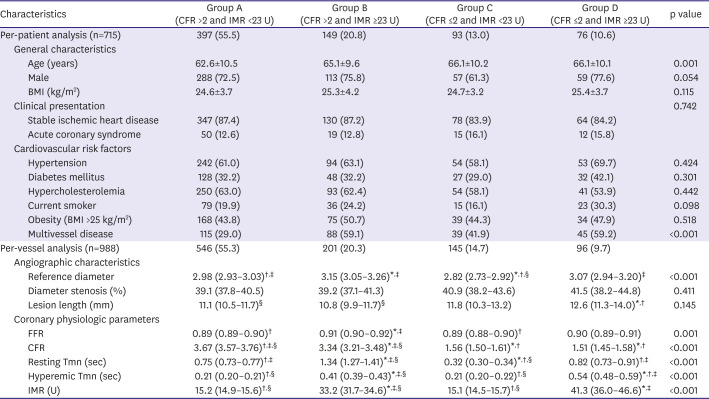
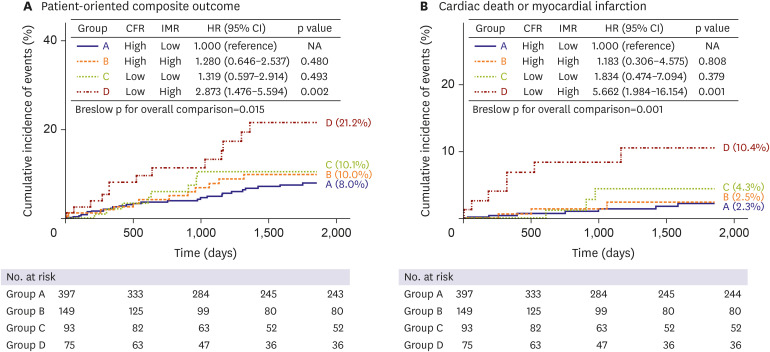
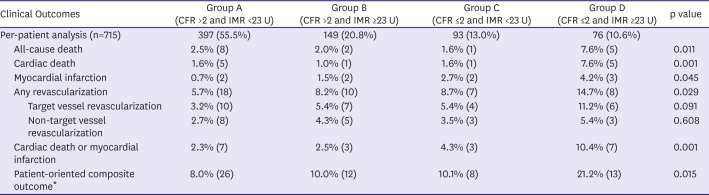
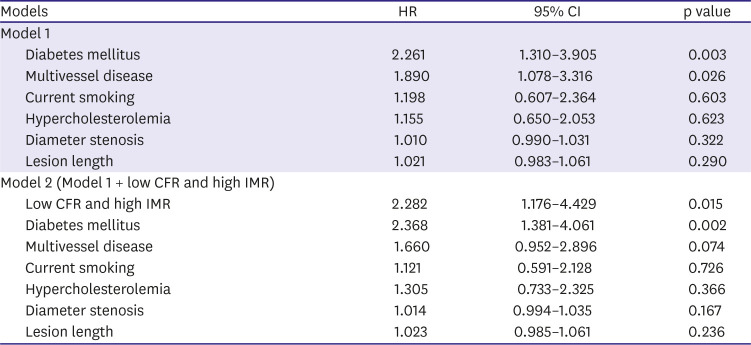




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download