INTRODUCTION
Transcatheter aortic valve replacement (TAVR) procedures have been performed more frequently worldwide as its indications have expanded. Historically, TAVR was performed in patients with prohibitive or high surgical risk. In contrast, it is now performed in low risk patients with severe symptomatic aortic stenosis (AS).
1)2)3)4)5)6) In Korea, the TAVR procedure is poorly reimbursed by national insurance; regardless, the number of TAVR procedures is on the constant rise.
7) The latest version of the balloon-expandable TAVR valve, or Sapien 3 (S3) transcatheter heart valve (THV), became available in Korea in 2014. The S3 valve is now widely used in most Korean TAVR centers.
The S3 THV was developed with several advanced features to overcome the drawbacks of its predecessor, the Sapien XT (SXT). The valve frame was coated with an outer polyethylene terephthalate skirt to reduce significant paravalvular leakage (PVL), as PVL predicts poor long-term prognosis.
8) In addition, the S3 THV has a flared inflow morphology, which might contribute to the lower PVL in the S3 than that in the SXT.
9) The required minimal luminal diameters of the femoral access site were further minimalized by the slender profile of the delivery system. Therefore, the transfemoral approach can be more generously applied for TAVR candidates with peripheral artery disease. Periprocedural bleeding complications are expected to be lower in the S3 THV than with SXT.
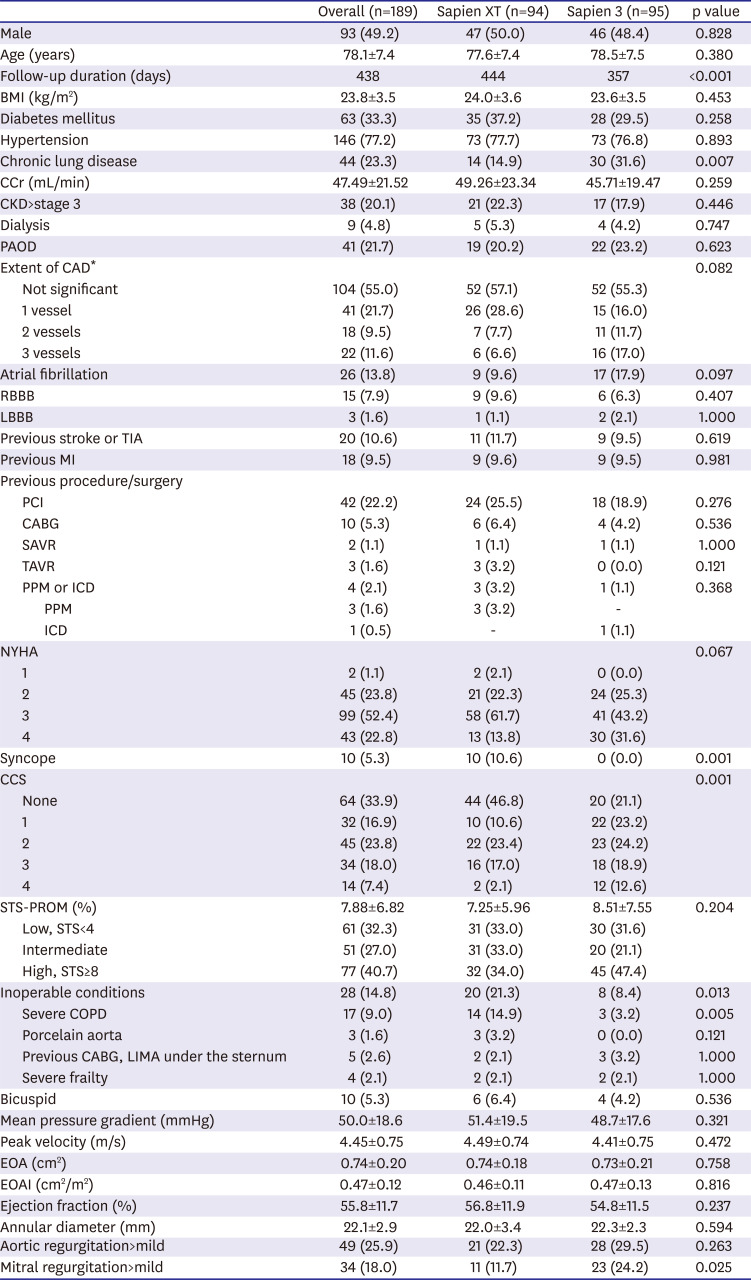
However, the overlaid outer skirt of the S3 THV might influence the effective orifice area (EOA) within the implanted THV due to its own thickness. Therefore, a prosthesis-patient mismatch may occur more frequently in patients treated with the S3 THV than it does with SXT. Asians tend to have a smaller annulus than do western people. Therefore, this theoretical prosthesis-patient mismatch is more likely to occur when THVs of the same size are implanted in Asian patients than it does in other populations. However, it is not well known how these prosthesis-patient mismatch problems relate to clinical results after TAVR. Therefore, we compared the procedural and clinical outcomes between Korean patients treated with S3 vs. SXT THV.
DISCUSSION
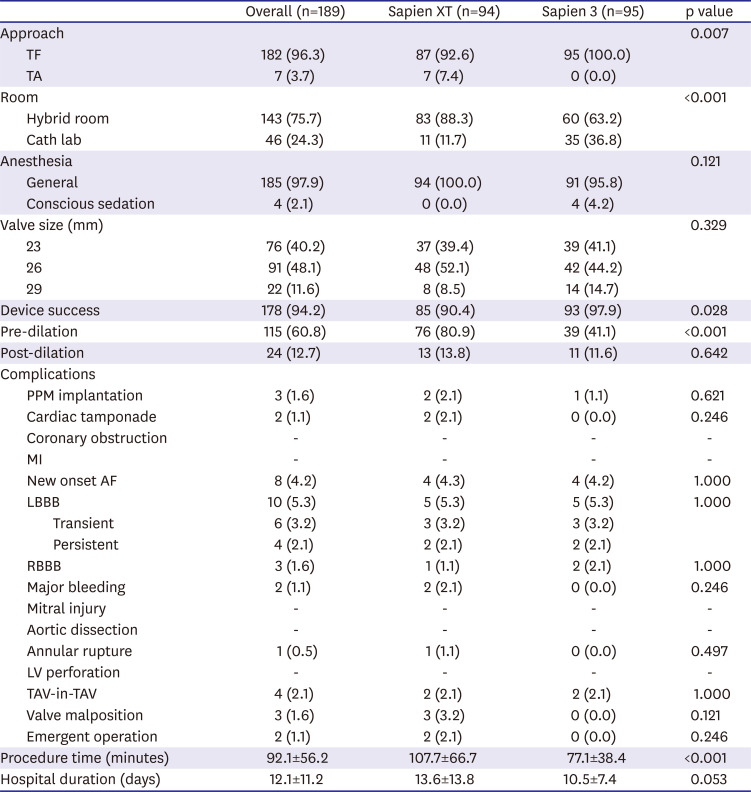
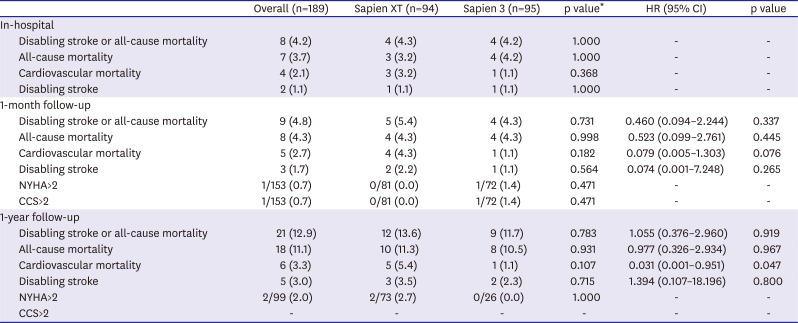
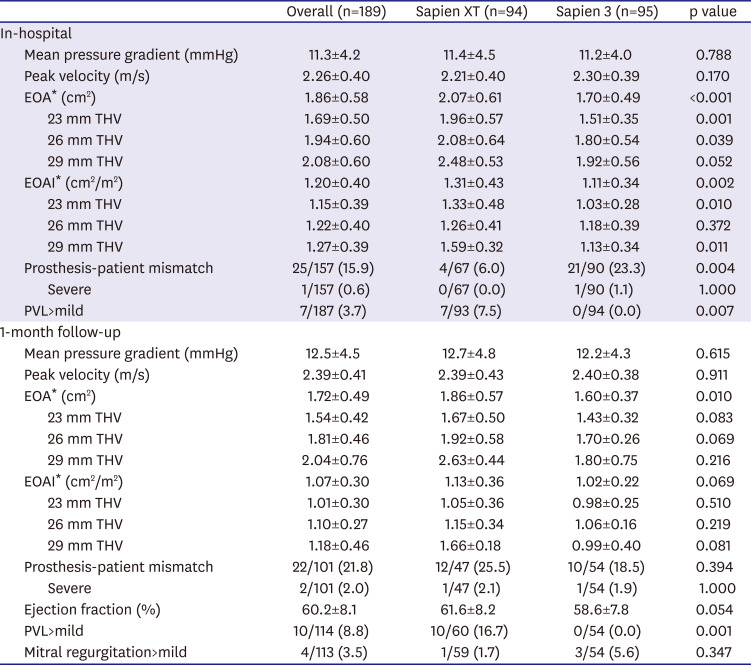
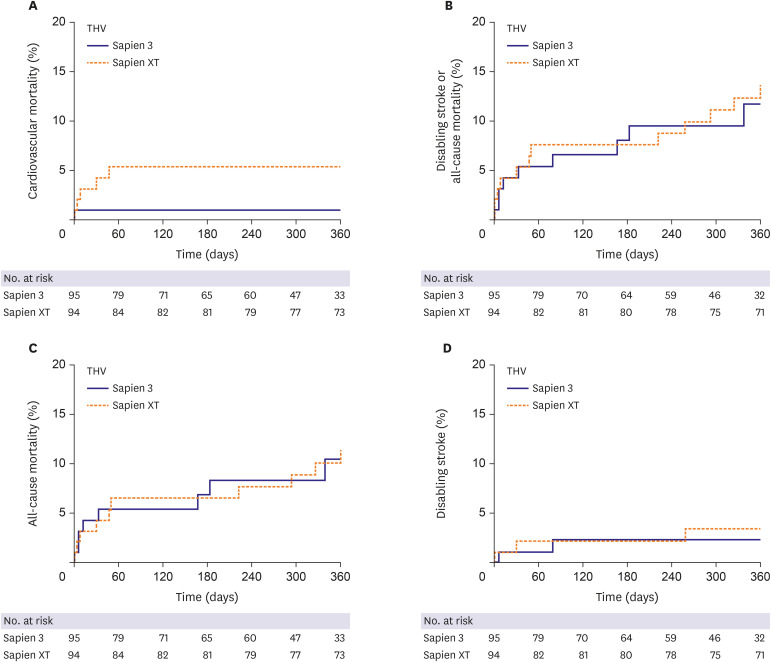
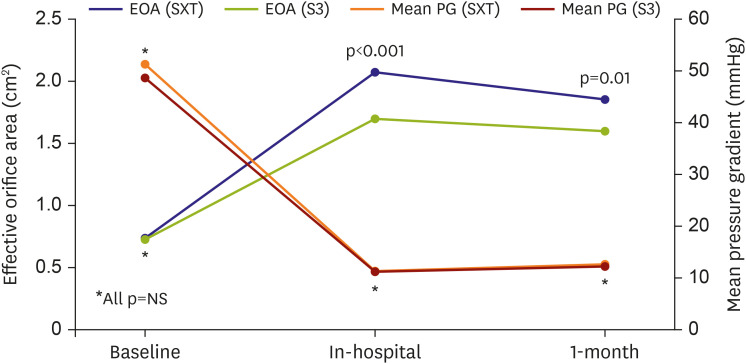
In this study, we compared the procedural and clinical outcomes between the SXT group and the S3 group. The major findings of the present study are as follows: 1) cardiovascular mortality rate at 1 year was significantly lower in the S3 group than it was in the SXT group (5.4% vs. 1.1%; HR, 0.031; 95% CI, 0.001–0.951; p=0.047); 2) TAVR using the S3 THV produced a significantly higher device success rate than did the SXT THV (SXT 90.4% vs. S3 97.9%; p=0.028); 3) the higher device success rate in the S3 group was mainly driven by a reduction in moderate or severe PVL (7.5% vs. 0.0%; p=0.007); 4) the EOA was significantly smaller in the S3 group during the in-hospital period (2.07±0.61 vs. 1.70±0.49 cm2; p<0.001) and at 1 month (1.86±0.57 vs. 1.60±0.37 cm2; p=0.010) than it was in the SXT group; 5) hospital stay (12.1±11.2 days) was longer than in the previously reported K-TAVI registry (7.5±6.1 days), probably due to different study periods (2010–2018 in the present study and 2015–2017 in K-TAVI registry) and difference in severity of patient characteristics (STS PROM≥8, 40.7% in the present study, 34.7% in K-TAVI registry).
TAVR performed using the S3 THV showed a significantly higher device success rate than that of the SXT THV. This success rate was mainly driven by a remarkable reduction in the PVL in the S3 group. These findings are consistent with those of previous reports.
13)14) Significant PVL is a well-known, long-term prognostic factor after TAVR implantation.
15)16) In the early period of TAVR, the prevalence of moderate to severe PVL was reported to be >10%.
17) The risk factors for PVL are not fully understood; however, some of the known contributing factors include prosthesis under-sizing, device malpositioning, and uneven aortic annular and valve leaflet calcifications. Recent studies have shown that 3D-reconstructed computed tomography allows for accurate measurement of aortic root structures for TAVR procedures.
18) In addition to the precise measurement of aortic structures and enhanced experience with the TAVR procedure, a polyethylene terephthalate outer skirt of the S3 THV contributed to the remarkable reduction of PVL in this study.
Also, in this study, the rate of pre-TAVR balloon valvuloplasty was much lower in the S3 group than the SXT group (SXT 80.9% vs. S3 41.1%; p<0.001). One explanation for this phenomenon is that balloon sizing was more frequently utilized in the early days of TAVR experience. As 3D-reconstructed computed tomography becomes more precise, the need for pre-TAVR balloon valvuloplasty appears to have diminished.
All TAVR procedures in the S3 group were performed via the transfemoral route, because the low profile of the e-sheath (of the S3) allows for the use of small vessels as the access site. However, there were no differences between the 2 groups with regard to major bleeding event rates or vascular access site complications. Other peri-procedural complications, such as PPM implantation rate, were similar between the 2 groups. Several recent studies have reported that S3 THV was associated with a higher rate of PPM implantation and new-onset intraventricular conduction abnormalities than is SXT, which is in contrast to our results. This discrepancy may be explained by low implantation due to the different foreshortening length of the valve platform, which develops during valve deployment. Based on increasing experience using the S3 TAVR, there does not appear to be a higher incidence of conduction abnormalities than that using the SXT. Also, in our study, PPM implantation rate (1.6%) and incidence of new-onset LBBB (5.3%) after TAVR are lower than those of previous studies (PPM rate in PARTNER trials 3.4–8.5%, CoreValve trials 11.7–25.9%) and of K-TAVI registry data (PPM rate 5.6%, new-onset LBBB data not available)
3)4)7)19)20). One possible explanation for these differences is that the present study only included balloon-expandable TAVR devices, which are known to have lower likelihood of atrioventricular block after TAVR. In K-TAVI registry data, about half of the study population was treated with self-expandable valve. Also, lower incidence of pre-existing bundle branch block (LBBB 1.6%, RBBB 7.9%) than that of a previous study (LBBB 3.0%, RBBB 10.3% in PARTNER 3 trial) may result in lower incidence of new PPM implantation.
5) In addition, smaller sizes of THV used in TAVR might contribute to the low incidence of conduction disorder.
21) In present study, up to 90% of patients were treated with THV size no greater than 26 mm. Among 10 patients who developed LBBB, 4 had persistent LBBB, while 6 recovered from bundle branch block. This was similar to the results of previous studies, which have shown that more than half of these conduction abnormalities disappear within days to months following TAVR with a balloon-expandable valve.
22)
The one-month and one-year clinical outcomes were acceptable and similar to those previously described in high surgical risk patients.
23) There was no significant difference in incidence of all-cause mortality or disabling stroke between the 2 groups. This result is in line with previous findings.
23) Cardiovascular mortality rate at 1 year was significantly lower in the S3 group than it was in the SXT group. Multivariable Cox regression analysis demonstrated that S3 was the only protective factor for cardiovascular mortality at 1 year (HR, 0.031; 95% CI, 0.001–0.951; p=0.047), while pre-existing atrial fibrillation was a risk factor (HR, 22.933; 95% CI, 2.153–244.270; p=0.009;
Supplementary Table 1). The S3 group had a higher incidence of NYHA IV heart failure, Canadian Cardiovascular Society grade IV angina, atrial fibrillation, and multi-vessel coronary artery disease than did the SXT group. The mean STS-PROM score was also numerically higher in the S3 group than it was in the SXT group. Nevertheless, cardiovascular mortality was lower in the S3 group than it was in the SXT group. The difference in cardiovascular mortality occurred within 60 days of the procedure and was maintained for one year (
Figure 2A). Possible explanations for this early difference in cardiovascular mortality include cumulative TAVR experience, a higher device success rate, enhanced cooperation of the heart team, and post-procedural management in the S3 group.
On the other hands, inverse probability of treatment weighting adjusted analysis and propensity score matching analysis showed no significant difference in incidence of cardiovascular mortality between the 2 group. The most reliable explanation for these conflicting results between multivariable Cox regression analysis and other analysis methods is believed to be due to the small number of study subjects, large number of covariates to match or adjust, and lack of sufficient clinical events due to relatively short-term follow-up. Therefore, the association between device generation and cardiovascular mortality observed in this study should be reconfirmed in large-scale future studies with longer-term follow-up period.
In present study, the EOA and EOAI after TAVR were significantly smaller in the S3 group than they were in the SXT group. In contrast, body mass index, annular diameter, and size of THV were not different between the 2 groups. One of the best explanations for the differences in EOA and EOAI between 2 groups is the difference in thickness of a polyethylene terephthalate outer skirt of the S3 THV. This finding provides some insight regarding the possibility of a prosthesis-patient mismatch and longevity of newer generation balloon-expandable THV.
Prosthesis-patient mismatches occur when the EOA of the prosthesis is smaller than patient body surface area.
24) A smaller EOA may lead to a patient-prosthesis mismatch, which could give rise to hemodynamic alterations and have a negative effect on valve durability. A severe prosthesis-patient mismatch might also impact patient long-term survival and functional recovery.
25) Prosthesis-patient mismatches are considered to be severe when EOAI is <0.65 cm
2/m
2, while an EOAI >0.85 cm
2/m
2 is not significant.
12) One small prospective study in France showed that the S3 group had small EOA and EOAI, and that there was more frequent prosthesis-patient mismatch rate in the S3 group compared to that with SXT.
26) Prosthesis-patient mismatch was most frequent in the 23-mm-sized S3 THV in the study.
In this study, the mean EOAI in the S3 group was generally larger than the definition of a severe prosthesis-patient mismatch (in-hospital mean EOAI 1.11±0.34 cm2/m2, 1 month 1.02±0.22 cm2/m2 in the S3 group). However, patients with significant prosthesis-patient mismatch must be carefully monitored for signs of poor valve longevity and functional aggravation. The post-TAVR mean pressure gradient of S3 valve was very low and not different from that of the SXT valve. Following from this, a smaller EOA in the S3 group does not seem to deteriorate hemodynamics after TAVR. Although a smaller EOA is unlikely to affect short-term clinical outcomes, we are unable to predict long-term outcomes due to the absence of relevant data. However, we tolerate the (not clinically significant) small EOA of the S3 group because the S3 group otherwise demonstrated a higher device success rate, lower PVL rate, and lower cardiovascular mortality rate than did the SXT group.
The number of valve-in-valve TAVR procedures for failed bioprosthetic surgical aortic valves has increased with acceptable efficacy and safety.
27)28) In valve-in-valve procedures, prosthesis-patient mismatch issues are very important, because this procedure creates a relatively smaller EOA than does conventional TAVR.
29) This is particularly true with small valve sizes. Valve-in-valve procedures with SXT may provide a larger EOA than do those with S3. Therefore, patients with a prosthesis-patient mismatch may be better served with valve-in-valve procedures using SXT instead of S3. Therefore, SXT may have a role in this particular setting if PVL risk is not high.
Clinical implications in present study we would like to emphasize are as follows. 1) The benefit of reducing PVL due to the outer skirt may be accompanied by a trade-off of reduced EOA/EOAI. 2) A THV such as SXT without an outer skirt may be advantageous in patients with low risk of PVL, that is, with less calcium in the aortic structure or those undergoing a valve-in-valve procedure. 3) Therefore, we believe that diversifying the portfolio of balloon-expandable valves will help optimize the clinical outcome of various patients with severe AS. For example, in a patient group where the risk of PVL is low and the securing of EOA is important, such as valve-in-valve, THV without outer skirt may be more advantageous.
This study has several limitations. First, it has all of the inherent limitations of a small, retrospective study design. Since the sample size and the number of events in this study were relatively small, all confounding factors could not be adequately corrected. A second limitation is that there was a time difference in the SXT and the S3 eras. Therefore, intra-operator experience between early period and late period could not be corrected adequately and these might have influenced the clinical outcomes. Third, we are lacking data regarding oversizing % and implantation depth of THV.
In conclusion, cardiovascular mortality was lower in the S3 group than in the SXT group in patients with severe symptomatic AS treated with balloon-expandable TAVR over 1 year of follow-up. The S3 THV demonstrated a higher device success rate and lower significant PVL rate than did the SXT THV. The long-term impact of a smaller EOA of S3 THV on clinical outcomes and the longevity of valve function remain to be determined in future studies.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download