Although contemporary cardiovascular treatment strategies including antiplatelet agents and statins have improved the clinical outcomes in high-risk patients with atherosclerotic cardiovascular disease (ASCVD), the mortality rate from ischemic heart disease remains unchanged and up to 5% of patients suffered from recurrent CV events each year.
1) Therefore, novel therapeutic strategies are required to prevent adverse clinical events.
2)3)
The development of direct oral anticoagulants (DOACs) has stimulated interest in studying the important role of the coagulation cascade in atherosclerotic progression and occurrence of atherothrombotic events.
4)5)6) Factor Xa and thrombin play critical roles during platelet activation and fibrin formation. The latter properties are essential for stable platelet-fibrin clot generation at the site of vascular injury during ischemic event occurrences (
Figure 1).
2)3) In addition, these components are also closely associated with all stages of development and progression of atherosclerosis. Cell signaling at the site of atherosclerotic plaque is mediated through the activation of protease-activated receptor (PAR).
2)3) PARs are expressed on diverse cell membranes including endothelial cells, leukocytes, platelets, and vascular smooth muscle cells (SMCs). PAR-mediated signaling is involved in endothelial cell activation and dysfunction, the inflammatory process by stimulating the generation of pro-inflammatory cytokines and chemokines, and proliferation and apoptosis of vascular SMCs. Factor Xa activates PAR-1 and PAR-2, whereas thrombin activates PAR-1, PAR-3, and PAR-4. Therefore, reducing factor Xa/thrombin levels with DOAC or inhibiting platelet activation through the PAR-1 antagonist may have a potential to attenuate the atherosclerosis progression and subsequent ischemic event occurrences.
4)5)6) Clinical trials using these antithrombotic agents already have shown clinical benefit in reducing the risk of ASCVD.
2)3)
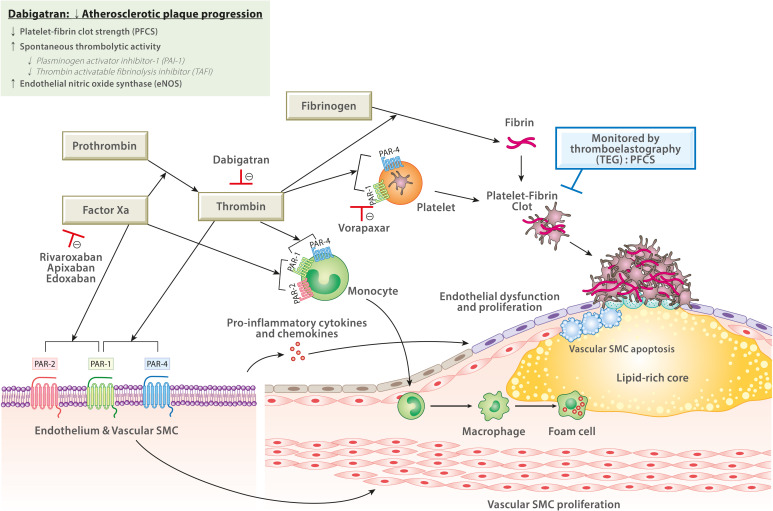 | Figure 1
Role of factor Xa and thrombin in progression of atherothrombosis (modified from Capodanno et al. and Gurbel et al.).2)3)
Factor Xa and thrombin contribute to atherothrombotic events through various mechanisms. Factor Xa (via PAR-1 and PAR-2) and thrombin (via PAR-1 and PAR-4) regulate the activation of endothelial cells, leukocytes, platelets, and vascular SMCs. PAR-mediated signaling are involved in endothelial cell activation and dysfunction, inflammatory process by production of pro-inflammatory cytokines and chemokines, and proliferation and apoptosis of vascular SMCs. Sustained inflammation in the lesion induces plaque instability and promotes plaque rupture. Thrombin is critical for platelet activation (via PAR-1 and PAR-4) and fibrin formation, which contribute to platelet-fibrin clot formation after plaque rupture. This experimental study showed that dabigatran has a potential to decrease progression of atherosclerotic plaque through reducing PFCS, and increasing spontaneous thrombolytic activity and eNOS.
eNOS = endothelial nitric oxide synthase; PAI-1 = plasminogen activator inhibitor-1; PAR = protease-activated receptor; PFCS = platelet-fibrin clot strength; SMC = smooth muscle cell; TAFI = thrombin-activatable fibrinolysis inhibitor; TEG = thromboelastography.

|
In this edition of the
Korean Circulation Journal, Sanda et al.
7) reported the interesting evaluation regarding thrombin inhibitory effect and spontaneous thrombolytic activity by dabigatran in apolipoprotein E (ApoE)
−/−–low-density lipoprotein receptor (LDLR)
−/− double-knockout mice. Their study showed that thrombin inhibition by dabigatran reduced atherosclerotic plaque progression and increased thrombolytic activity.
7) Following thrombin inhibition, expressions of plasminogen activator inhibitor-1 (PAI-1) and thrombin-activatable fibrinolysis inhibitor (TAFI) were decreased, but endothelial nitric oxide synthase (eNOS) was increased. Results of the present study were similar with previous experimental studies using direct thrombin inhibitors,
2)3) which may suggest a close association between the activity of the coagulation system and atheroma progression.
Coronary artery disease (CAD) is a pathological process characterized by atherosclerotic plaque generation in the epicardial arteries.
8) The atherosclerotic disease can have long, stable periods but can become unstable at any time, infrequently resulting in an acute clinical event caused by plaque rupture or erosion. This dynamic nature of the coronary atherothrombotic process can be categorized as either acute coronary syndrome (ACS) or chronic coronary syndrome (CCS). To maintain stability of CCS, pharmacological therapies are essential. Platelet activation and aggregation is the main trigger for symptomatic arterial thrombosis, forming the foundation for long-term use of antiplatelet agents in CCS patients. Dual antiplatelet therapy (DAPT) with aspirin and an oral P2Y
12 inhibitor is the mainstay of antiplatelet therapy in patients with myocardial infarction (MI).
2)3) Anticoagulant agents such as DOAC inhibit the generation of thrombin, which plays a pivotal role in both coagulation and platelet activation. However, dual pathway inhibition (DPI) with antiplatelet therapy and standard-dose anticoagulant (e.g., warfarin or apixaban) for post-ACS secondary prevention was associated with an unfavorable balance of efficacy and bleeding. The recent clinical trials have generated interest in DPI use with antiplatelet therapy and low-dose DOAC (e.g., rivaroxaban 2.5 mg twice a day) in CCS patients with high-risk features.
8)9)
Current guidelines recommend adjunctive use of P2Y
12 inhibitor (e.g., clopidogrel, prasugrel or ticagrelor) or vascular-dose DOAC in addition to aspirin among patients who have moderate-to-high ischemic risk without high bleeding risk (
Table 1).
8) It would be questionable whether post-MI patients with additional risk factors can be treated with DPI or prolonged DAPT. There are unmet needs to develop the scoring system or surrogate biomarker(s) for guiding optimal antithrombotic strategies in these patients. Several studies have already demonstrated that ASCVD patients have higher thrombin concentration compared with those without ASCVD.
2)3) Our group has evaluated clinical usefulness of thromboelastography (TEG) assay in ASCVD patients. This assay provides information on fibrin and thrombin generation, platelet aggregation, the interaction between fibrin and platelets to form a stable platelet-fibrin clot, and the contribution of platelet count/function and fibrinogen activity to strength and stability of the clot.
6) Platelet-fibrin clot strength (PFCS) measured by TEG showed a close relationship with on-clopidogrel platelet reactivity and combining the measurements of platelet aggregation and PFCS enhanced the risk stratification of major clinical events in CAD patients undergoing percutaneous coronary intervention (PCI).
4) In addition, the level of PFCS was associated with the atheroma burden in CAD patients. In PCI-treated patients (n=1,667), the presence of peripheral artery disease (PAD) was significantly related with elevated levels of C-reactive protein and PFCS.
5) Furthermore, high PFCS was significantly associated with worse clinical outcomes only in PAD patients.
5)
Table 1
Treatment options for dual antithrombotic therapy in combination with low-dose aspirin in patients*

|
Drug option |
Dose |
Indication |
Additional cautions |
|
Clopidogrel |
75 mg od |
Post-MI in patients who have tolerated DAPT for 1 year |
|
|
Prasugrel |
10 mg od (5 mg od if BW <60 kg or age >75 years) |
Post-PCI in MI patients who have tolerated DAPT for 1 year |
Age >75 years |
|
Ticagrelor |
60 mg bid |
Post-MI in patients who have tolerated DAPT for 1 year |
|
|
Rivaroxaban |
2.5 mg bid |
Post-MI >1 year or multivessel CAD |
Creatinine clearance 15–29 mL/min |

Additional risk factors associated with high ischemic risk (e.g., multivessel CAD, PAD, heart failure, diabetes mellitus, chronic kidney disease, and recurrent MI) in MI patients seemed to have a close relationship with the activity of coagulation pathway.
4)5)6) Inhibition of thrombin cascade with vascular-dose DOAC in these patients may have a greater potential to attenuate the progression of atherosclerotic lesion and the risk of atherothrombotic event occurrence. For example, the sub-analysis of Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease (COMPASS) trial including patients with lower-extremity PAD or carotid artery disease demonstrated that vascular-dose rivaroxaban (2.5 mg twice daily) plus aspirin markedly lowered the incidence of major adverse limb events by 43%, major vascular amputation by 67%, and peripheral vascular intervention by 24% compared with aspirin monotherapy.
9)
Exploring an optimal dose of DOAC in East Asian patients may be another important research topic. During antithrombotic therapy, East Asian patients have shown a lower risk for ischemic events and a higher risk for bleeding, especially in the gastrointestinal and central nervous system (“East Asian Paradox”).
10) Moreover, individual DOAC has been shown to have different pharmacokinetic profiles according to the race. The active metabolite of rivaroxaban and dabigatran were 20–30% higher in East Asian compared with Caucasians.
10) In the COMPASS trial, a regimen of 2.5 mg of rivaroxaban twice daily increased major bleeding by 1.2% in Caucasians (aspirin 2.2% vs. rivaroxaban plus aspirin 3.4%) and 2.1% in Asians (aspirin 1.8% vs. rivaroxaban plus aspirin 3.9%), respectively.
9) Our recent analysis suggested that East Asians may have different level of coagulation activity in patients with stable CAD.
6) East Asians showed a lower level of PFCS (61.8±7.9 vs. 65.4±5.0 mm, p<0.001) and a lower prevalence of high PFCS (defined as ≥68 mm) (odds ratio [OR], 0.50; 95% confidence interval [CI], 0.27–0.93; p=0.028) compared with Caucasians. High PFCS was significantly associated with major CV events (adjusted OR, 6.27; 95% CI, 2.41–16.30; p<0.001). With different levels of coagulation activity (e.g., thrombin concentration) and PFCS, the optimal dose of DOAC to achieve net clinical benefit may different according to the race. The time has come to accept the concept of precision medicine based on ethnicity and surrogate biomarker(s) that strongly associated with individual intrinsic property for ischemia and bleeding (
Figure 1). Accordingly, the use of optimal-potency DOAC may be another strategy to achieve stabilization or regression of atherothrombotic disease and prevent ischemic event occurrences in CCS patients.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download