This article has been
cited by other articles in ScienceCentral.
Abstract
Background and Objectives
Severe aortic stenosis (AS) with left ventricular systolic dysfunction (LVSD) is a class I indication for aortic valve replacement (AVR) but this recommendation is not well established in those at the stage of moderate AS. We investigate the clinical impact of AVR among patients with moderate AS and LVSD.
Methods
From 2001 to 2017, we consecutively identified patients with moderate AS and LVSD, defined as aortic valve area 1.0–1.5 cm2 and left ventricular ejection fraction <50%. The primary outcome was all-cause death. The outcomes were compared between those who underwent early surgical AVR (within 2 years of index echocardiography) at the stage of moderate AS versus those who were followed medically without AVR at the outpatient clinic.
Results
Among 255 patients (70.1±11.3 years, male 62%), 37 patients received early AVR. The early AVR group was younger than the medical observation group (63.1±7.9 vs. 71.3±11.4) with a lower prevalence of hypertension and chronic kidney disease. During a median 1.8-year follow up, 121 patients (47.5%) died, and the early AVR group showed a significantly lower all-cause death rate than the medical observation group (5.03PY vs. 18.80PY, p<0.001). After multivariable Cox-proportional hazard regression adjusting for age, sex, comorbidities, and laboratory data, early AVR at the stage of moderate AS significantly reduced the risk of death (hazard ratio, 0.43; 95% confidence interval 0.20–0.91; p=0.028).
Conclusions
In patients with moderate AS and LVSD, AVR reduces the risk of all-cause death. A prospective randomized trial is warranted to confirm our findings.
Keywords: Aortic stenosis, Heart valve prosthesis implantation, Heart failure, Prognosis
INTRODUCTION
Degenerative aortic stenosis (AS) is the most common valvular heart disease in elderly patients. Overall, AS affects 12% of patients older than 75 years, and severe AS affects 3% of those.
1) Patients with concomitant severe AS and left ventricular (LV) systolic dysfunction (LVSD) is a class I indication for aortic valve replacement (AVR) even in those without symptoms.
2)3) However, this recommendation is not well established in those with moderate AS and LVSD. Whether severe or not, LV wall stress increases by the hemodynamic afterload with AS and it may increase even more with LVSD,
4) thus aggravating the pump failure.
Moderate AS also significantly increases the risk of mortality compared with those without AS.
5) In addition, a grave clinical outcome of patients with moderate AS and LV dysfunction has been reported.
6) Theoretically, the relief of AS may improve the LV function and patient survival at the stage of moderate AS, with a few reports describing the beneficial hemodynamic effects of transcatheter AVR for these patients and a case of surgical AVR.
7)8) However, the early intervention for AS in those with LVSD has not been looked into systematically nor has the effect of early AVR on outcome been investigated.
We hypothesized that in patients with moderate AS and LVSD, early AVR at the stage of moderate AS would improve patient survival. The objectives of this study were 2-fold; first, to analyze the outcome of those with LVSD who underwent AVR at the stage of moderate AS and second, to compare this outcome with those who were followed medically.
METHODS
Study population
From August 2001 to December 2017, consecutive patients with moderate AS and concomitant LVSD, i.e. LV ejection fraction (LVEF) ≤50%, were identified from the echocardiography database in Seoul National University Hospital (SNUH). Moderate AS was defined primarily based on aortic valve area (AVA) 1.0–1.5 cm
2 and then double-checked by the maximal aortic valve (AV) velocity 2–4 m/s.
6) We excluded patients who had previously undergone a prior AV surgery or any type of AVR, whether surgical or transcatheter. There were no patients with congenital heart disease or hypertrophic cardiomyopathy. As the current guidelines recommend serial echocardiographic re-evaluation of a patient with moderate AS within 2 years,
2)3) we defined early AVR as an AVR that had been done within 2 years from the index echocardiography.
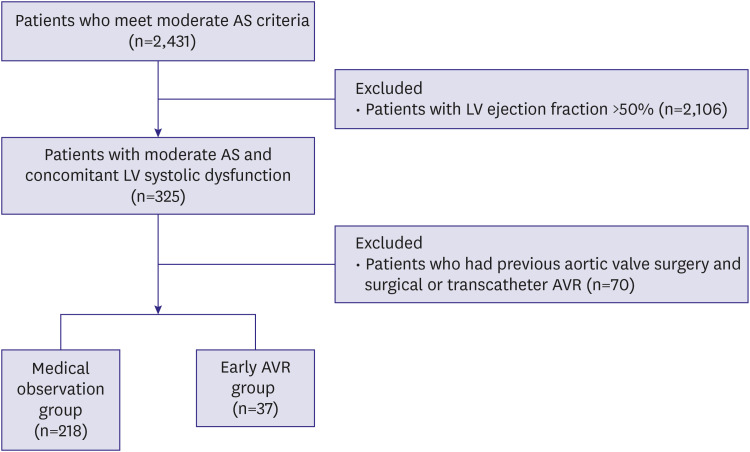
Figure 1 presents the overall study flow.
Figure 1
Study flow of the study participants.
AS = aortic stenosis; AVR = aortic valve replacement; LV = left ventricular.

Patient demographics, the New York Heart Association (NYHA) functional class, comorbidities, medication, and laboratory data were obtained from medical records. The comorbidities included hypertension, diabetes mellitus, dyslipidemia, prior percutaneous coronary intervention (PCI), prior coronary bypass graft surgery (CABG), chronic obstructive pulmonary disease (COPD), and a previous history of stroke. We also collected data on medication with special emphasis on those that are commonly prescribed for heart failure: beta-blocker, renin-angiotensin system (RAS) blocker, spironolactone, diuretics, digoxin, calcium channel blocker, and statin. The study protocol was approved by the SNUH Institutional Review Board (H-1901-170-1007) and patient consent was waived as this was a retrospective analysis of a consecutive cohort of patients.
Echocardiography
All echocardiographic studies were performed by experienced clinical sonographers using commercially available ultrasound equipment (Vivid 7; GE Healthcare, Chicago, IL, USA; i33; Philips, Amsterdam, Netherlands; or Sequoia; Siemens Medical Solutions, Malvern, PA, USA). Two-dimensional echocardiography, continuous- and pulsed-wave Doppler measurements were obtained using standard techniques and procedures according to the guidelines from the American Society of Echocardiography.
9) The AVA was calculated based on the continuity-equation using velocity-time integral measured at the AV and the LV outflow tract. The LVEF was determined by the modified quinones equation or the modified biplanar method.
Study endpoints
The primary endpoint of the study was all-cause death. The mortality and the cause of mortality were obtained from the Statistics Korea, the national statistical office of Korea. All International Statistical Classification of Diseases (ICD)-10-CM codes in the range of I00 to I99 were defined as cardiovascular death and death due to other ICD codes were categorized as non-cardiac death. Death without a definite ICD code was classified as death of unknown cause. Patients were followed until December 2017 as the mortality data from Statistics Korea only provides the data up to 2 years prior to the query.
Statistical analysis
Continuous variables are presented as mean±standard deviation and categorical variables as numbers (%). The entire population was divided into those who underwent early AVR versus those who did not and were followed medically. Student's t-test was used to compare the difference in the continuous variables, and the χ2 test or Fisher's exact test was used to compare the prevalence of categorical variables between the 2 groups. Survival free from all-cause or cardiovascular death was depicted by Kaplan-Meier survival curves and a log-rank test was used to analyze the difference in the survival between the early AVR versus the medical observation group. Survival analysis was done with the time from the index echocardiography. A Cox-proportional hazard regression model was adjusted for age, sex, LVEF, AVA, hypertension, diabetes mellitus, dyslipidemia, prior PCI, CABG, COPD, previous history of stroke, hemoglobin, creatinine, RAS blockade, and beta-blockade.
To reduce the treatment-selection bias and the potential confounding factors, we rigorously adjusted for significant differences in the characteristics of the 2 groups using the inverse probability of treatment weighting (IPTW) method. The IPTW uses the whole dataset and assigns an inverse probability of received treatment weighting by applying the 1/propensity score (PS) for patients in the treated cohort (early AVR group) and [1/(1−PS)] for those in the control cohort (medical observation group). The propensity for each treatment group was estimated using a logistic regression method, including all clinical variables that were used for adjustment in the Cox-proportional hazard regression model. An absolute standardized difference (ASD) of 0.1 or less is considered to balance each covariate between the 2 groups. The detail of the PS weighting results is shown in
Supplementary Table 1. For a sensitivity analysis, early AVR group was defined as patients who received AVR within 90 days of index echocardiography, and the impact of early AVR on all-cause death was analyzed using the same method described previously. Also, as a subgroup analysis, we excluded the patients who received cardiac surgery for reasons other than moderate AS, such as CABG or other valvular surgery, after the index echocardiography.
A p value of <0.05 was used to verify statistical significance. All statistical analyses were conducted using R version 3.4.3 (
http://www.r-projec-t.org).
RESULTS
Baseline characteristics comparison between the early aortic valve replacement versus the medical observation group
A total of 255 patients with moderate AS and concomitant LVSD were included in the analysis (
Table 1). Among these, 37 patients (14.5%) received AVR within 2 years of the index echocardiography (herein, defined as early AVR group) and 218 patients were followed medically (medical observation group). In the medical observation group, 11 patients received AVR at a time more than 2 years away from the index echocardiography (median 4.5 [interquartile range 3.2–6.6] years). The cumulative incidence of AVR in the entire patients is shown (
Supplementary Figure 1).
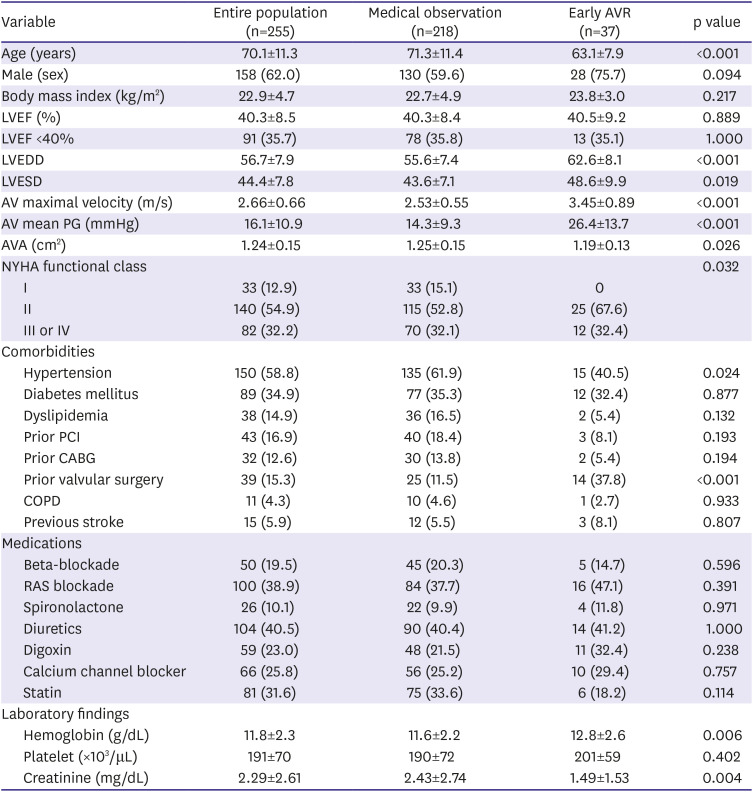
Table 1
Baseline characteristics in the early AVR group versus the medical observation group
|
Variable |
Entire population (n=255) |
Medical observation (n=218) |
Early AVR (n=37) |
p value |
|
Age (years) |
70.1±11.3 |
71.3±11.4 |
63.1±7.9 |
<0.001 |
|
Male (sex) |
158 (62.0) |
130 (59.6) |
28 (75.7) |
0.094 |
|
Body mass index (kg/m2) |
22.9±4.7 |
22.7±4.9 |
23.8±3.0 |
0.217 |
|
LVEF (%) |
40.3±8.5 |
40.3±8.4 |
40.5±9.2 |
0.889 |
|
LVEF <40% |
91 (35.7) |
78 (35.8) |
13 (35.1) |
1.000 |
|
LVEDD |
56.7±7.9 |
55.6±7.4 |
62.6±8.1 |
<0.001 |
|
LVESD |
44.4±7.8 |
43.6±7.1 |
48.6±9.9 |
0.019 |
|
AV maximal velocity (m/s) |
2.66±0.66 |
2.53±0.55 |
3.45±0.89 |
<0.001 |
|
AV mean PG (mmHg) |
16.1±10.9 |
14.3±9.3 |
26.4±13.7 |
<0.001 |
|
AVA (cm2) |
1.24±0.15 |
1.25±0.15 |
1.19±0.13 |
0.026 |
|
NYHA functional class |
|
|
|
0.032 |
|
I |
33 (12.9) |
33 (15.1) |
0 |
|
II |
140 (54.9) |
115 (52.8) |
25 (67.6) |
|
III or IV |
82 (32.2) |
70 (32.1) |
12 (32.4) |
|
Comorbidities |
|
|
|
|
|
Hypertension |
150 (58.8) |
135 (61.9) |
15 (40.5) |
0.024 |
|
Diabetes mellitus |
89 (34.9) |
77 (35.3) |
12 (32.4) |
0.877 |
|
Dyslipidemia |
38 (14.9) |
36 (16.5) |
2 (5.4) |
0.132 |
|
Prior PCI |
43 (16.9) |
40 (18.4) |
3 (8.1) |
0.193 |
|
Prior CABG |
32 (12.6) |
30 (13.8) |
2 (5.4) |
0.194 |
|
Prior valvular surgery |
39 (15.3) |
25 (11.5) |
14 (37.8) |
<0.001 |
|
COPD |
11 (4.3) |
10 (4.6) |
1 (2.7) |
0.933 |
|
Previous stroke |
15 (5.9) |
12 (5.5) |
3 (8.1) |
0.807 |
|
Medications |
|
|
|
|
|
Beta-blockade |
50 (19.5) |
45 (20.3) |
5 (14.7) |
0.596 |
|
RAS blockade |
100 (38.9) |
84 (37.7) |
16 (47.1) |
0.391 |
|
Spironolactone |
26 (10.1) |
22 (9.9) |
4 (11.8) |
0.971 |
|
Diuretics |
104 (40.5) |
90 (40.4) |
14 (41.2) |
1.000 |
|
Digoxin |
59 (23.0) |
48 (21.5) |
11 (32.4) |
0.238 |
|
Calcium channel blocker |
66 (25.8) |
56 (25.2) |
10 (29.4) |
0.757 |
|
Statin |
81 (31.6) |
75 (33.6) |
6 (18.2) |
0.114 |
|
Laboratory findings |
|
|
|
|
|
Hemoglobin (g/dL) |
11.8±2.3 |
11.6±2.2 |
12.8±2.6 |
0.006 |
|
Platelet (×103/μL) |
191±70 |
190±72 |
201±59 |
0.402 |
|
Creatinine (mg/dL) |
2.29±2.61 |
2.43±2.74 |
1.49±1.53 |
0.004 |

The early AVR group was younger than the observation group (63.1±7.9 vs. 71.3±11.4 years, p<0.001) and had a slightly higher proportion of male patients. The LVEF was similar in both groups but the LV dimension was significantly larger and the AVA smaller in the early AVR group. All patients in early AVR group had some degree of dyspnea, i.e. NYHA functional class II–IV, whereas 15% of the medical observation group did not have any dyspnea at the time of baseline echocardiography. The prevalence of comorbidities or the medication prescription patterns were not different between the 2 groups except for the prevalence of hypertension. The early AVR group showed a slightly higher level of hemoglobin and significantly better renal function than the medical observation group.
Clinical information on the early aortic valve replacement
The clinical characteristics of the 37 patients who underwent early AVR are summarized (
Table 2). Approximately two-thirds of the patients (n=25) had NYHA class II dyspnea at the time of AVR, whereas the remainder had dyspnea of either NYHA class III or IV. Most of the patients with NYHA class III or IV dyspnea were admitted via the emergency department and received AVR at the time of emergency visit.
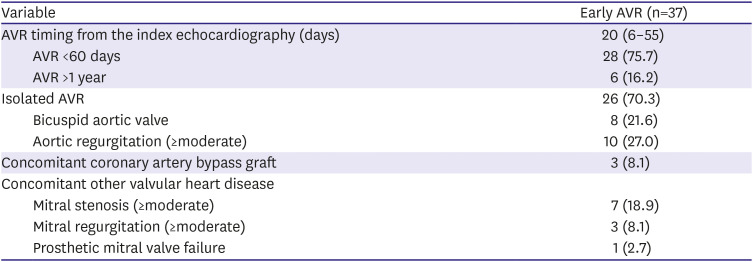
Table 2
Patient characteristics of the early AVR group
|
Variable |
Early AVR (n=37) |
|
AVR timing from the index echocardiography (days) |
20 (6–55) |
|
AVR <60 days |
28 (75.7) |
|
AVR >1 year |
6 (16.2) |
|
Isolated AVR |
26 (70.3) |
|
Bicuspid aortic valve |
8 (21.6) |
|
Aortic regurgitation (≥moderate) |
10 (27.0) |
|
Concomitant coronary artery bypass graft |
3 (8.1) |
|
Concomitant other valvular heart disease |
|
|
Mitral stenosis (≥moderate) |
7 (18.9) |
|
Mitral regurgitation (≥moderate) |
3 (8.1) |
|
Prosthetic mitral valve failure |
1 (2.7) |

The AVR was done at a median 20 days after the index echocardiography, with 75% of the entire early AVR done within 60 days from the index echocardiography. Isolated AVR was performed in 26 patients (70%), and 10 patients had concomitant significant aortic regurgitation of moderate degree or more. The most frequent concomitant valvular disease other than the AV was mitral stenosis, followed by mitral regurgitation. Concomitant CABG was done in less than 10% of the patients.
Impact of early aortic valve replacement on mortality
During a median 1.8 years (interquartile range, 0.6–4.6 years) follow-up, 121 patients (47.5%) died. In the latest follow-up echocardiography, defined as an echocardiography done at least 6 months after the index echocardiography, 42 patients (19.3%) in the medical observation group have a recovery of LVEF to >50%. In contrast, 22 patients (59.5%) of the early AVR group had a recovery of LVEF to >50%. As for the primary outcome, cardiovascular death was the most frequent cause of death and followed by malignancy, infection, diabetes mellitus, and chronic kidney disease (
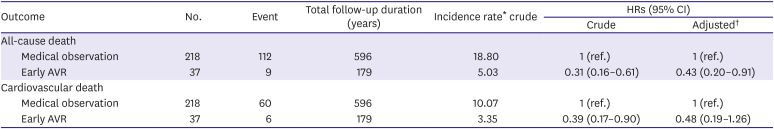
Supplementary Table 2). The incidence rate of all-cause death was significantly lower in the early AVR group compared with the medical observation group (
Table 3, 5.03 vs. 18.80 per 100 person-years, p<0.001). Early AVR was also associated with a reduced risk of cardiovascular death as well (
Table 3, p=0.027).
Table 3
Association of early AVR with all-cause or cardiovascular death of patients with moderate AS and concomitant LVSD
|
Outcome |
No. |
Event |
Total follow-up duration (years) |
Incidence rate* crude |
HRs (95% CI) |
|
Crude |
Adjusted†
|
|
All-cause death |
|
|
|
|
|
|
|
Medical observation |
218 |
112 |
596 |
18.80 |
1 (ref.) |
1 (ref.) |
|
Early AVR |
37 |
9 |
179 |
5.03 |
0.31 (0.16–0.61) |
0.43 (0.20–0.91) |
|
Cardiovascular death |
|
|
|
|
|
|
|
Medical observation |
218 |
60 |
596 |
10.07 |
1 (ref.) |
1 (ref.) |
|
Early AVR |
37 |
6 |
179 |
3.35 |
0.39 (0.17–0.90) |
0.48 (0.19–1.26) |

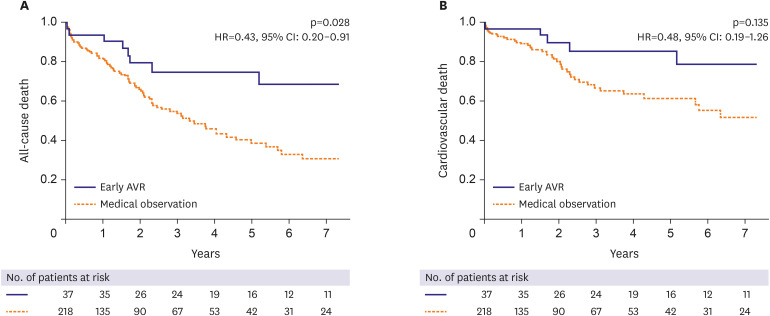
The early AVR was significantly associated with a reduction of the risk of all-cause death (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.20–0.91) in the multivariable-adjusted Cox proportional regression analysis (
Table 3,
Figure 2A). For cardiovascular death, the incidence was lower in the early AVR group; however, the early AVR was not associated with a significant reduction of the risk of cardiovascular death when adjusted for covariates (
Table 3,
Figure 2B).
Figure 2
Adjusted Kaplan-Meier survival curves for mortality according to early AVR within 2 years of index echocardiography. Comparison of mortality between the early AVR group versus the medical observation group after multivariable-adjusted Cox proportional regression analysis. (A) All-cause death. (B) Cardiovascular death.
AVR = aortic valve replacement; CI = confidence interval; HR = hazard ratio.

We used the IPTW method to reduce the differences between the 2 groups; however, some covariates were still unbalanced after the IPTW (
Supplementary Table 1). Nevertheless, early AVR was associated with a reduced risk of all-cause death after the IPTW, and early AVR still reduced the risk of all-cause death when further adjusted for clinical factors by the multivariate Cox-proportional regression in this IPTW cohort (HR, 0.70; 95% CI, 0.49–0.99;
Supplementary Table 3).
As a sensitivity analysis, we redefined early AVR as AVR that was done within 90 days of the index echocardiography, to minimize the possibility that early AVR may, in fact, be done at the stage of severe AS. Again, early AVR that was done within 90 days also reduced the risk of all-cause death (
Supplementary Table 4). In the subgroup with patients who underwent isolated early AVR, the AVR was associated with a risk reduction of all-cause death as well (
Supplementary Table 5).
DISCUSSION
In this study, we tested the hypothesis of whether early AVR at the stage of moderate AS would improve patient survival in patients with moderate AS and LVSD. With approximately 50% of mortality during a median 2 years follow-up, 15% of the study population underwent early AVR. Our main finding is that early AVR is associated with a risk reduction of all-cause death, thus suggesting that earlier relief of the LV afterload in pressure-overloaded heart may be helpful for improving survival.
Per current guidelines, AVR for moderate AS may be considered concomitantly when the patient is planned to undergo CABG, surgery for ascending aorta, or surgery for another valve.
2)3) However, there is a large lack of evidence that supports this recommendation and furthermore, there is nearly no evidence that supports isolated AVR in those with moderate AS and LVSD. Studies up to now that provided support for the recommendations in the current guidelines did not focus on LVSD.
10)11)12) Because the incidence of heart failure and AS increases with age,
6)13) more interest in and understanding of these patients with moderate AS and LVSD should be needed.
It is well known that AS increases afterload to the LV, thus increasing the LV wall stress.
14) Patients with low myocardial contractility already have high LV wall stress, which may further worsen with even moderate AS, and in the long-term, worse survival.
15)16)17) A recent report by Ito et al.
4) has demonstrated that a subset of patients have LVSD even at a stage of moderate AS, with worse survival in those with LVEF <50% and also, 50–60%. Considering the current status of AVR in moderate AS and LVSD, these findings suggest that there remain significant rooms for improvement of survival in these patients. Although the potential semantic benefit of AVR can be inferred based on these findings from the previous publications, the literatures published so far is quite limited. A recent case study of a patient with moderate AS and LVSD reported a beneficial hemodynamic change after the transcatheter AVR, with a left-shifted pressure-volume curve, improved myocardial contractility and oxygen consumption.
7)
So far, only a few studies have reported a beneficial clinical outcome in patients who underwent early AVR at the stage of moderate AS. Recently, Samad et al. analyzed patients with moderate or severe AS and LVSD from the Duke Echocardiographic Laboratory Database.
18) The AVR was associated with a 35% reduction of mortality in these patients, a finding that was similar to our study. It is interesting to note that compared to the previous publications, only 15% of the entire population underwent AVR in our study population within 2 years of the diagnosis and 25% of the population in other publications underwent early AVR within the same time period. Previous studies have defined the AVR group as patients who underwent AVR within 5 years of the index echocardiography. We wanted to be sure that the AVR was done at the moderate stage and therefore, the early AVR group was defined as those who received AVR within 2 years of the index echocardiography, which is a time interval recommended for follow-up in moderate AS.
2)3) Additionally, there was a lack of laboratory and drug information in the previous study and we provided more evidence that the early AVR was indeed associated with a risk reduction of mortality, with rigorous adjustment of the possible covariates. Although the periprocedural risk should be considered when planning any intervention, these findings suggest that the relief of AS even at the moderate stage in patients with LVSD may significantly improve the clinical outcome. It also adds to the justification of an ongoing prospective randomized trial to directly address the effect of AVR in moderate AS and LVSD, the Transcatheter Aortic Valve Replacement to Unload the Left ventricle in patients with ADvanced heart failure trial (
NCT02661451).
19)
This study is not without limitations. First, the differences in baseline characteristics between the 2 groups may confound the results. To reduce these imbalances, we performed the IPTW method; unfortunately, the ASD of some covariates were still >0.1. However, early AVR was associated with a risk reduction of all-cause death after multivariate Cox-proportional regression in the IPTW cohort. Second, this study was a single-center retrospective cohort and the patient population was relatively small. There may be potential unknown confounders beyond the factors that we adjusted. Patients in the early AVR group had slightly higher AV velocity and smaller AVA than medical observation group. There could be a concern of the physician's selection bias for performing the AVR. Even so, the difference of survival between the 2 groups after adjustment suggests that the AVR could be a considerable option for these patients. Furthermore, with the introduction of transcatheter AVR recently, the patients deemed unsuitable for surgical AVR could be referred to transcatheter AVR. Third, the cause of LVSD could be multifactorial; however, the prevalence of prior PCI or CABG was not different between the groups and the benefit of early AVR was still significant after adjustment of these factors. Fourth, the measurement error could influence the severity of AS, and additional examinations, such as dobutamine stress echocardiography and/or computed tomography may help to improve the diagnostic accuracy. In this study, only 4 patients in the medical observation group and 2 patients in the early AVR group performed dobutamine stress echocardiography, however, we believe that the AVA can be reliably measured with echocardiography in skillful hands. Finally, the guideline-recommended medication for heart failure was not well implemented with less than 50% of the patients using the appropriate medications. However, there was no significant difference in the medication patterns between both groups.
In conclusion, early AVR at the stage of moderate AS is associated with a significant improvement of survival in patients with moderate AS and LVSD. Although the study population of our study is relatively small and may not be enough to give a conclusive answer to this clinical enigma, our findings await a definite answer by the upcoming prospective randomized trials in the near future as well as suggesting that there is room for survival improvement for these patients.








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download