This article has been
cited by other articles in ScienceCentral.
Abstract
Background and Objectives
In East Asia, tuberculous pericarditis still occurs in immunocompetent patients. We aimed to investigate clinical course of tuberculous pericarditis and the trends of echocardiographic parameters for constrictive pericarditis.
Methods
We retrospectively analyzed medical records of patients with tuberculous pericarditis between January 2010 and January 2017 in Samsung Medical Center. Treatment consists of the standard 4-drug anti-tuberculosis regimen for 6 months with or without corticosteroids. We performed echocardiography at initial diagnosis, 1, 3 and 6 months later.
Results
Total 50 cases with tuberculous pericarditis in immunocompetent patients were enrolled. Echocardiographic finding at initial diagnosis divided into 3 groups: 1) pericardial effusion only (n=28, 56.0%), 2) effusive constrictive pericarditis (n=10, 20.0%) and 3) constrictive pericarditis (n=12, 24.0%). The proportion of patients with constrictive pericarditis decreased gradually over time. After 6 months, only 5 patients still had constrictive pericarditis. Out of the 28 patients who initially presented with effusion alone, only one patient developed constrictive pericarditis. Echocardiographic parameters representing constrictive pericarditis gradually disappeared over the follow up period. Ventricular interdependency improved significantly from 1 month follow-up, whereas septal bounce and pericardial thickening were still observed after 6 months without significant constrictive physiology.
Conclusions
Tuberculous pericarditis with pericardial effusion without constrictive physiology is unlikely to develop into constrictive pericarditis in immunocompetent hosts, if treated with optimal anti-tuberculous medication and steroid therapy. Even though there were hemodynamic feature of constrictive pericarditis, more than 80% of the patients were improved from constrictive pericarditis.
Keywords: Tuberculous pericarditis, Constrictive pericarditis, Echocardiography, Steroids
INTRODUCTION
Between 20–40% of all treated cases of tuberculous pericarditis evolve to chronic constrictive pericarditis.
1)2) Tuberculosis remains the leading cause of constrictive pericarditis throughout the world, especially in endemic area. The mortality rate of untreated acute effusive tuberculous pericarditis is nearly 85%.
3)4)
Most of previous studies of tuberculous pericarditis have focused on immunocompromised patients, such as human immunodeficiency virus (HIV)-infected patients. In East Asia where
Mycobacterium tuberculosis is endemic, however, tuberculous pericarditis still occurs in immunocompetent patients. While constrictive pericarditis has been traditionally presumed to be irreversible, previous studies have described reversible constrictive pericarditis. In this “transient” constrictive pericarditis, constrictive physiology and hemodynamics resolve without surgical intervention.
5) Tuberculous pericarditis can potentially be a type of transient constrictive pericarditis
6)7); however, reversibility is not always guaranteed before treatment is completed.
7)
The efficacy of concomitant steroid therapy during anti-tuberculosis treatment remains controversial.
7) Several studies of patients with effusive constrictive tuberculous pericarditis have concluded that tuberculostatic treatment combined with steroids might be associated with fewer deaths and a less frequent need for pericardiocentesis or pericardiectomy, although these differences were not statistically significant.
8)9) The echocardiographic features of tuberculosis pericarditis vary from pericardial effusion to effusive constrictive pericarditis or established constrictive pericarditis,
10) and clinical course of tuberculous pericarditis might differ according to echocardiographic features. However, no comprehensive echocardiographic follow-up study of tuberculous pericarditis has yet been performed.
In this study, we investigated the clinical course of tuberculous pericarditis and the changes in echocardiographic parameters that assess constrictive pericarditis during treatment of tuberculous pericarditis in immunocompetent patients.
METHODS
Study population
The clinical pathway for treatment and follow-up of tuberculosis pericarditis was established during 2010 at our hospital (Samsung Medical Center, Seoul, Republic of Korea). Therefore, we retrieved data from all patients who were coded with tuberculosis pericarditis in our electrical medical record system between January 2010 and January 2017. Patients who were older than 19 years and who were diagnosed with tuberculous pericarditis were included in this study if they had undergone serial echocardiography according to the clinical pathway, i.e. at baseline, 1 month, 3 months and 6 months of follow-up. Patients with immunocompromised status such as concomitant HIV-infection, cancer or taking immunosuppressants were excluded.
Data collection
Demographic data, comorbidities, initially presenting symptoms were collected from patient hospital records. Patients who have been treated for pulmonary tuberculosis or who had an apical scar change on their chest X-ray were considered to have a history of pulmonary tuberculosis. The presence of concurrent extracardiac tuberculosis was also investigated. In terms of treatment, the 4-drug anti-tuberculosis regimen for 6 months was defined as standard therapy (standard isoniazide, rifampin, ethambutol, and pyrazinamide for 2 months followed by maintenance 4 months of isoniazide and rifampin). Prolonged treatment was considered as treatment for more than 6 months. Adjunctive corticosteroid therapy and the need for drainage of pericardial effusion were also assessed. Corticosteroid therapy was not used in the case of relative contraindication such as old age or combined viral disease, and terminated early when side effects occurred. Pericardial culture results for M. tuberculosis, pericardial fluid smear microscopy results for acid fast bacilli, pericardial biopsy results and initial laboratory test (including pericardial effusion analysis) were also collected.
Definition
Diagnostic criteria were used to classify patients as having definite or probable tuberculous pericarditis. Definite diagnosis of tuberculous pericarditis was based on the presence of M. tuberculosis in pericardial fluid or tissue by culture, microscopy or PCR (Xpert MTB/RIF) testing. Probable diagnosis was made when there was other evidence for tuberculosis in patients with unexplained pericarditis, e.g. lymphocytic pericardial exudate with elevated interferon-gamma (INF-γ) >50 pg/mL, adenosine deaminase (ADA) >40 U/L or caseating granulomas identified on biopsy of pericardium or other tissue such as lymph node.
Transthoracic echocardiography (TTE)
TTE was performed at baseline, 1 month, 3 months, and 6 months after the initial treatment. Image acquisition and measurements were performed according to the American Society of Echocardiography guideline.
11) Images were stored in DICOM format and electronically transferred to the Echocardiographic Core Laboratory (ECL) at Samsung Medical Center. Final measurements and analysis were performed at ECL with a dedicate software package, EchoPAC (GE Healthcare, Chicago, IL, USA).
Ventricular interdependence during respiration and septal bouncing were evaluated in mid-short axis and apical 4-chamber view. Mitral and tricuspid inflows were measured at the opening of mitral and tricuspid leaflets from the 4-chamber view under concomitant respirometry. Diastolic flow reversal of the hepatic vein (HV) at expiration was measured from the subcostal window. In addition, the septal and lateral mitral annulus early diastolic velocities (septal e′ and lateral e′) were determined by tissue Doppler at the level of the mitral septal annulus and lateral annulus.
12) The presence and amount of pericardial effusion was measured. Hemodynamic evaluation of cardiac tamponade and nature of the pericardial effusion (clear or complicated) were described. Respiratory variation of the inferior vena cava (IVC) and its diameter were assessed in the subcostal view.
Constrictive physiology was diagnosed echocardiographically by either: 1) the septal mitral annular e′ velocity greater than lateral mitral annular e′, together with thickened pericardium; or 2) a clear, visually determined respirophasic shift of the interventricular septum towards the left ventricular cavity during inspiration in an apical four-chamber view.
When diffusely thickened pericardium, increased pericardial brightness, visceral pericardial thickening and irregularities along with pericardial effusion were observed, we considered pericardial thickening.
Statistical analysis
Numerical data are presented with median and interquartile range values or with mean ± standard deviation. Categorical data are presented with total sample size (n) and relative (%) frequencies. We visually investigated and estimated rank correlation statistics (Somers' D) to verify the nature and order of improvement, as measured by various echocardiographic parameters, after which we performed a formal test using the Cochran-Armitage trend test statistic. All analyses were performed in R statistical software, and p values <0.05 were considered statistically significant.
RESULTS
Sixty-nine patients were diagnosed with tuberculous pericarditis between January 2010 and January 2017. Nineteen patients were excluded from the final analysis because of missing echocardiographic follow-up (n=9), diagnostic error during clinical follow-up (n=6) or immunocompromised status (n=4). The medical records and echocardiographic data of 50 patients for whom serial echocardiographic measurements were available were finally reviewed.
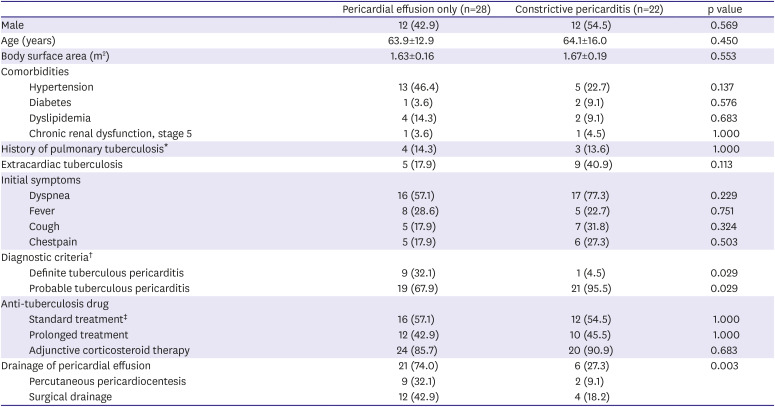
The clinical characteristics of the study population are summarized in
Table 1. The mean age was 64 years old and there was no gender difference. Extracardiac tuberculosis was present in 17 patients (34.0%), 10 of whom had pulmonary tuberculosis and 7 of whom had extra-pulmonary tuberculosis consisting of extrathoracic lymphadenopathy or prosthetic joint infection. The most common symptom at initial presentation was dyspnea (n=33, 66.0%) followed by fever, cough and chest pain. In terms of treatment, all patients were treated with standard anti-tuberculosis treatment for more than 6 months and most of patients (n=44, 88.0%) were treated with corticosteroids. Pericardial effusion drainage was performed in 32 patients (64.0%) and consisted of percutaneous drainage (n=11) or surgical drainage (n=21).
Table 1
Clinical characteristics according to initial echocardiographic diagnosis
|
Pericardial effusion only (n=28) |
Constrictive pericarditis (n=22) |
p value |
|
Male |
12 (42.9) |
12 (54.5) |
0.569 |
|
Age (years) |
63.9±12.9 |
64.1±16.0 |
0.450 |
|
Body surface area (m2) |
1.63±0.16 |
1.67±0.19 |
0.553 |
|
Comorbidities |
|
|
|
|
Hypertension |
13 (46.4) |
5 (22.7) |
0.137 |
|
Diabetes |
1 (3.6) |
2 (9.1) |
0.576 |
|
Dyslipidemia |
4 (14.3) |
2 (9.1) |
0.683 |
|
Chronic renal dysfunction, stage 5 |
1 (3.6) |
1 (4.5) |
1.000 |
|
History of pulmonary tuberculosis*
|
4 (14.3) |
3 (13.6) |
1.000 |
|
Extracardiac tuberculosis |
5 (17.9) |
9 (40.9) |
0.113 |
|
Initial symptoms |
|
|
|
|
Dyspnea |
16 (57.1) |
17 (77.3) |
0.229 |
|
Fever |
8 (28.6) |
5 (22.7) |
0.751 |
|
Cough |
5 (17.9) |
7 (31.8) |
0.324 |
|
Chestpain |
5 (17.9) |
6 (27.3) |
0.503 |
|
Diagnostic criteria†
|
|
|
|
|
Definite tuberculous pericarditis |
9 (32.1) |
1 (4.5) |
0.029 |
|
Probable tuberculous pericarditis |
19 (67.9) |
21 (95.5) |
0.029 |
|
Anti-tuberculosis drug |
|
|
|
|
Standard treatment‡
|
16 (57.1) |
12 (54.5) |
1.000 |
|
Prolonged treatment |
12 (42.9) |
10 (45.5) |
1.000 |
|
Adjunctive corticosteroid therapy |
24 (85.7) |
20 (90.9) |
0.683 |
|
Drainage of pericardial effusion |
21 (74.0) |
6 (27.3) |
0.003 |
|
Percutaneous pericardiocentesis |
9 (32.1) |
2 (9.1) |
|
Surgical drainage |
12 (42.9) |
4 (18.2) |

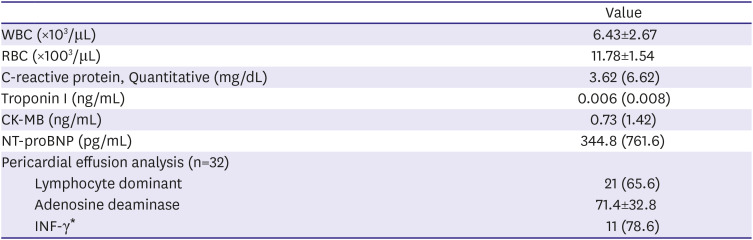
Pericardial effusion analysis was performed in 32 patients, of whom 21 (65.6%) showed lymphocyte-dominant exudate. The mean concentration of ADA in pericardial effusion was 71.4±32.8 U/L. While the pericardial INF-γ was measured in only 14 patients, 11 (78.6%) tested positive for INF-γ (
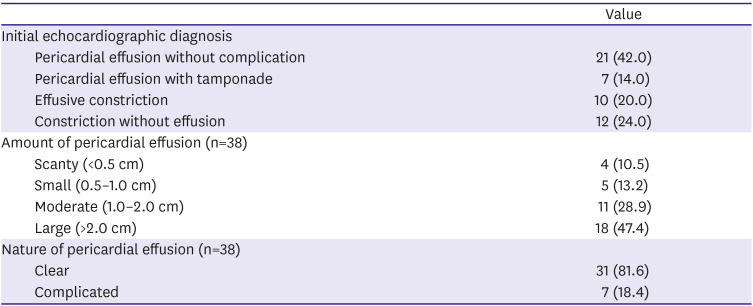
Table 2). The patients with tuberculous pericarditis presented with various clinical features on echocardiography at the first diagnosis. Based on the initial echocardiographic diagnosis, the patients were divided into three groups: 1) pericardial effusion only (n=28, 56.0%), 2) effusive constrictive pericarditis (n=10, 20.0%) and 3) constrictive pericarditis (n=12, 24.0%) (
Table 3). Patients presenting with pericardial effusion appeared to have large amount of pericardial effusion, as shown in
Table 3 (n=18, 47.4%).
Figure 1 displays the representative echocardiographic features of these 3 groups.
Figure 1
Initial transthoracic echocardiography. (A) Pericardial effusion without constriction. Large amount of pericardial effusion was noticed (bidirectional arrow). The septal mitral annulus early diastolic velocity (septal e′) was 0.049 m/s (closed arrow). The lateral mitral annulus early diastolic velocity (lateral e′) was 0.078 m/s (open arrow). There was no diastolic flow reversal of the HV. (B) Pericardial effusion with constriction. Small amount of pericardial effusion was observed with pericardial thickening. The septal e′ (0.082 m/s) was higher than the lateral e′ (0.070 m/s). Expiratory diastolic flow reversal of HV was noticed (open arrowhead). (C) Constrictive pericarditis without pericardial effusion. Pericardial thickening was seen (closed arrowhead). The septal e′ (0.164 m/s) was higher than the lateral e′ (0.104 m/s). And also expiratory diastolic flow reversal of HV was noticed (open arrowhead).
HV = hepatic vein.
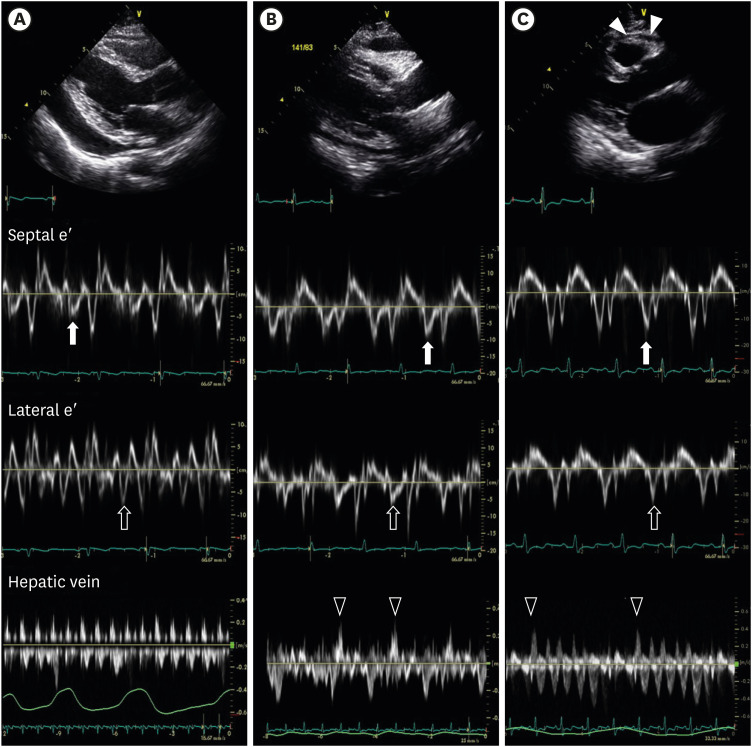

Table 2
Laboratory test at initial diagnosis (n=50)
|
Value |
|
WBC (×103/μL) |
6.43±2.67 |
|
RBC (×1003/μL) |
11.78±1.54 |
|
C-reactive protein, Quantitative (mg/dL) |
3.62 (6.62) |
|
Troponin I (ng/mL) |
0.006 (0.008) |
|
CK-MB (ng/mL) |
0.73 (1.42) |
|
NT-proBNP (pg/mL) |
344.8 (761.6) |
|
Pericardial effusion analysis (n=32) |
|
|
Lymphocyte dominant |
21 (65.6) |
|
Adenosine deaminase |
71.4±32.8 |
|
INF-γ*
|
11 (78.6) |

Table 3
Initial echocardiography (n=50)
|
Value |
|
Initial echocardiographic diagnosis |
|
|
Pericardial effusion without complication |
21 (42.0) |
|
Pericardial effusion with tamponade |
7 (14.0) |
|
Effusive constriction |
10 (20.0) |
|
Constriction without effusion |
12 (24.0) |
|
Amount of pericardial effusion (n=38) |
|
|
Scanty (<0.5 cm) |
4 (10.5) |
|
Small (0.5–1.0 cm) |
5 (13.2) |
|
Moderate (1.0–2.0 cm) |
11 (28.9) |
|
Large (>2.0 cm) |
18 (47.4) |
|
Nature of pericardial effusion (n=38) |
|
|
Clear |
31 (81.6) |
|
Complicated |
7 (18.4) |

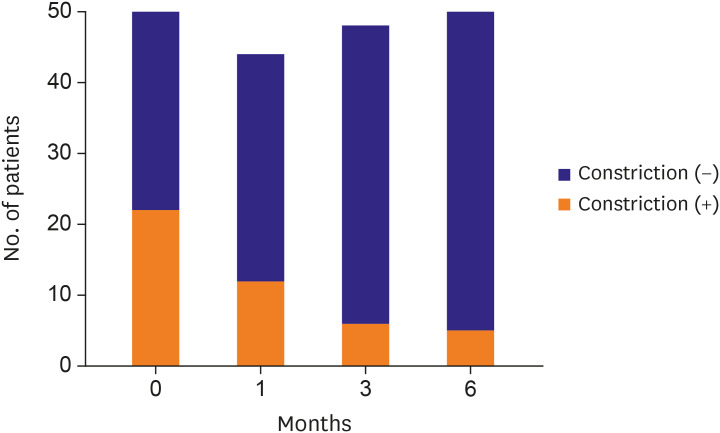
The proportion of patients with constrictive pericarditis decreased gradually over time (
Figure 2). At the time of diagnosis, 22 patients (44.0%) showed constrictive pericarditis, whereas only 12 patients (27.3%) showed constrictive pericarditis after 1 month. After 6 months, only 5 patients (10%) still had constrictive pericarditis.
Supplementary Table 1 shows baseline characteristics according to the residual constriction after 6 months.
Figure 2
Proportion of constrictive pericarditis over time. The proportion of patients with constriction decreased through the time. At initial diagnosis, 22 patients (44%) had constrictive pericarditis. After 1 month, the number of patients with constrictive pericarditis was reduced to 12 (27.3%) among 44 patients who underwent echocardiography. Six patients (12.5%) had constrictive pericarditis at 3 months and 5 patients (10%) remained chronic constrictive pericarditis at the end of 6 months for follow-up.

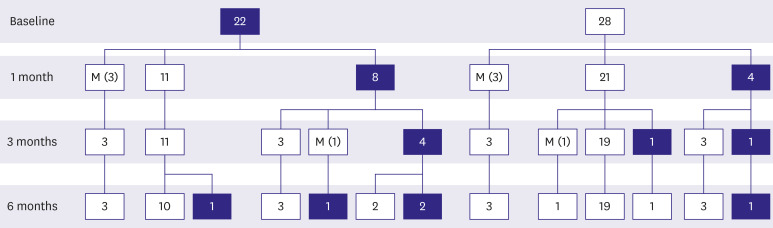
At initial diagnosis, 22 patients had tuberculous pericarditis with constrictive pericarditis with/without effusion (
Figure 3). After 1 month, half of the patients (n=11, 50%) had recovered from constrictive pericarditis, while constrictive physiology persisted in 8 patients. After 6 months of treatment, only 4 patients (18.2%) showed residual constriction. Among the 28 patients who did not show constrictive physiology at the initial diagnosis, 4 patients (14.3%) presented constriction at 1 month. Out of the 28 patients who initially presented with effusion alone, only one patient developed residual constrictive pericarditis (3.6%).
Figure 3
Clinical course of tuberculous pericarditis. The numbers in blue filled boxes mean the number of patients with constriction at that time. And the numbers in white boxes indicate the number of patients without constriction. Missing data is denoted by M (the number of patients). After 6 months of treatment, residual constrictive pericarditis developed in 4 patients (18.2%) among the 22 patients who initially presented constrictive pericarditis. However, only 1 patient developed residual constrictive pericarditis (3.6%) among 28 patients who initially presented effusion without constriction.

The echocardiographic parameters representing constrictive pericarditis gradually disappeared over the follow up period.
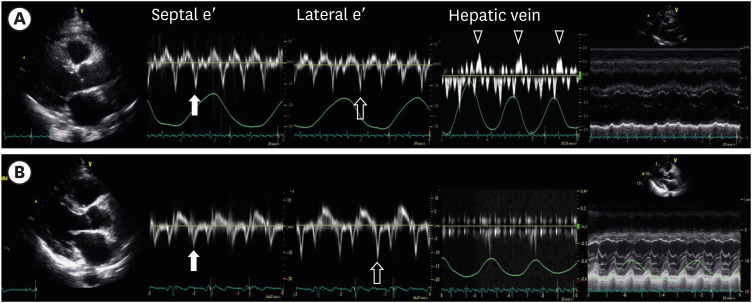
Figure 4 demonstrates the echocardiographic images before and after treatment. At baseline, a thickened pericardium, a paradoxical increase of septal e′ compared to lateral e′, expiratory diastolic flow reversal of HV, and ventricular interdependence were noted. After 6 months of medication combined with steroid treatment, all these findings had disappeared.
Figure 4
Resolution of constrictive pericarditis. (A) Initial echocardiography showed constrictive pericarditis with pericardial thickening (closed arrowhead). The septal mitral annulus early diastolic velocity (septal e′) was 0.136 m/s (closed arrow). The septal e′ was higher than the lateral e′ (0.116 m/s, open arrow). Expiratory diastolic flow reversal of the HV was observed dominantly (open arrowhead). In the M mode, ventricular interdependence was prominent. (B) After 6 months, the patients repeated echocardiography. Constrictive pericarditis was resolved. The septal e′(closed arrow) was 0.075 m/s which was lower than the lateral e′ of 0.120 m/s(open arrow). Expiratory diastolic flow reversal of HV was not observed. And also, ventricular interdependence disappeared.
HV = hepatic vein.

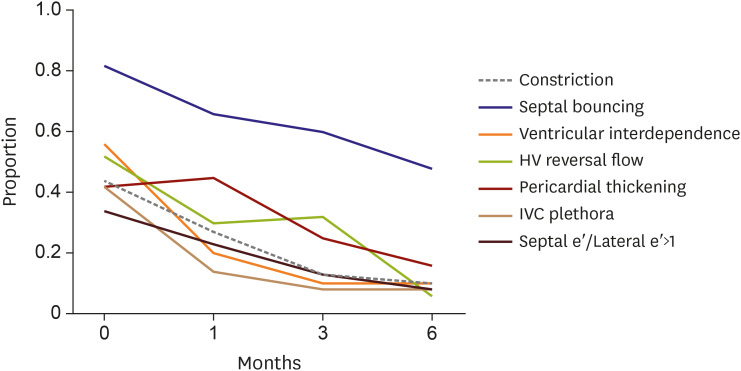
In the overall population, the changes in echocardiographic parameters varied. Ventricular interdependence appeared to be the earliest marker changing to resolution status from constrictive physiology (
Figure 5). Although the hemodynamic parameters improved in 90% of the study population, pericardial thickening remained in a few cases (10%) without significant constrictive physiology.
Figure 5
Echocardiographic parameters for constriction over time. Ventricular interdependence was the initial parameter of improvement. Septal bouncing and pericardial thickening still remained even after constriction improved.
HV = hepatic vein; IVC = inferior vena cava.

DISCUSSION
Tuberculous pericarditis is a potentially fatal disease and frequently progresses to constrictive pericarditis. However, there is no prognostic indicator to accurately determine who will remain with constrictive pericarditis after anti-tuberculous treatment. This is the first comprehensive echocardiographic study to include serial echocardiographic examination, including hemodynamic parameters.
From the clinical features at initial diagnosis, the absence of constrictive physiology rarely progressed to constrictive pericarditis after 6 months of anti-tuberculous treatment with 3 months of steroid therapy (3%). On the other hand, a higher proportion of patients remained with constrictive pericarditis (18%) in those initially presenting with constrictive pericarditis, even though they were treated with same regimen, including steroid therapy. Thus, constrictive pericarditis improved in more than 80% of the patients treated with anti-tuberculous medication and steroid therapy, even though hemodynamic features of constrictive pericarditis were present. These results indicate that the presence of pericardial effusion without constriction is a good prognostic indicator.
In previous studies without echocardiographic information, 20% to 40% of the patients diagnosed with tuberculous pericarditis finally progressed to constrictive pericarditis with optimal treatment. Moreover, if left untreated, 50% of the patients with effusive tuberculous pericarditis progressed to constriction.
2) Because our study population included non-HIV patients, the clinical course may be different. It may be possible that the immunocompetent patients in our study were diagnosed earlier than HIV-infected patients because of their better socioeconomic status, leading to earlier visits to the hospital. The presence of transient constrictive pericarditis has also been reported. Tuberculous pericarditis is also a cause of transient constrictive pericarditis, but prediction of reversibility is difficult when assessed with clinical features.
5)6)
Only one report has been published about echocardiographic features of tuberculous pericarditis for prognosis,
13) and the only echocardiographic feature included was pericardial effusion echogenicity. Many patients with pericardial effusion were included and about half of the patients who had constrictive physiology, including effusive constrictive pericarditis and constrictive pericarditis, finally remained with constrictive pericarditis. Of particular note, steroid therapy was prescribed for only half of the patients. In our study, about 90% of the patients were treated with steroid therapy. In particular, steroid therapy was prescribed for 20 out of the 22 patients with constrictive physiology at initial diagnosis. Moreover, even in the patients with constrictive physiology at initial diagnosis, the constrictive physiology resolved over time. This observation suggests that steroid therapy in tuberculous pericarditis contributes to the resolution of constrictive physiology at initial presentation. Furthermore, some patients developed constrictive physiology that resolved after treatment, suggesting that anti-tuberculous medication can elicit the same immune- reaction as that in tuberculous pleurisy, with a paradoxical response.
Previous studies of co-treatment of high dose steroid tuberculous pericarditis yield discrepant results, potentially because of low power of the clinical studies or their indeterminate results.
14)15)16) Considering that tuberculous pericarditis has various clinical features at its initial presentation, the previous results for steroid therapy are reasonable. Moreover, it is possible that a subgroup of patients without constrictive pericarditis at initial diagnosis can avoid unnecessary steroid therapy with close clinical monitoring with echocardiography.
Since the echocardiographic parameters for constrictive pericarditis vary and are not present in all cases, integration of hemodynamic parameters is important for diagnosis. During follow-up, we observed gradual changes of the hemodynamic features suggestive of constrictive pericarditis. Ventricular interdependence, which can be observed by 2-dimensional echocardiography, was the first parameter to show improvement. The proportion of septal bouncing was high and the septal bouncing persisted over follow up; pericardial thickening persisted the longest. Since septal bouncing and pericardial thickening often persisted even after constrictive pericarditis improvement, it is advisable to consider ventricular interdependence and diastolic flow reversal of the HV at expiration as early indicators for improving constrictive pericarditis before completion of treatment.
Occasional cases with constrictive pericarditis from baseline are observed. The cases involving transient occurrence in any follow-up period are shown in
Figure 3. After pericardiocentesis or surgical drainage, transient constrictive pericarditis can occur. It is also possible that constrictive pericarditis can be improved or worsened by changing the steroid dosage and/or duration. When physicians reduced the steroid dose for patients with tuberculosis pericarditis with constriction, they confirmed the deterioration of constrictive pericarditis by follow-up echocardiography. After the steroid dosage was increased again, improvement of constrictive pericarditis was observed in these patients. Serial echocardiography should be performed with steroid dose control.
Our study has several limitations. While the data for this study were derived from electrical medical records and an echocardiographic database system, the clinical review was retrospective. Furthermore, the number of patients was insufficient to perform statistical modeling to identify echocardiographic prognostic indicators predictive of the absence of future constrictive pericarditis. While our study points to suggestions for steroid therapy, further prospective studies for image-guided steroid therapy are needed.
In conclusion, tuberculous pericarditis with pericardial effusion without constrictive physiology is unlikely to develop into constrictive pericarditis in most of cases, if treated with optimal anti-tuberculous medication and steroid therapy. Tuberculous pericarditis with constrictive physiology at initial diagnosis can be reversible in 80% if the appropriate steroid treatment is given. According to serial echocardiography, ventricular interdependence was the earliest parameter of improvement in serial echocardiographic monitoring. Assessment of echocardiographic features in tuberculous pericarditis can provide prognostic information and help predict treatment response.











 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download