INTRODUCTION
Vascular injury is mainly characterized by migration to the intima and excessive proliferation of vascular smooth muscle cells (SMCs), resulting in thickening of the intima and neointimal formation.
1) Therapeutic strategies to ameliorate the progression of atherosclerosis have investigated the inhibition of SMC proliferation and migration using animal models of arterial injury.
2)3)
Compelling evidence has revealed that focal adhesions play critical roles in cell proliferation and migration.
4)5) Talin is one of the proteins that is found in high concentrations in focal adhesions. Talin binds to the cytoplasmic domain of integrin β subunit, and activates and links integrins to the actin cytoskeleton.
6)7) Upon integrin activation, talin recruits other focal adhesion and signaling proteins to the focal adhesion sites.
8) A previous study has revealed that depletion of talin using small hairpin RNA resulted in the absence of vinculin, paxillin, and focal adhesion kinase (FAK) at the focal adhesion sites.
9) FAK is another key component that also interacts with integrins, and is involved in integrin-mediated signal transduction.
10) FAK is a non-receptor tyrosine kinase that contains a focal adhesion-targeting (FAT) domain, which binds to talin and paxillin.
11) Autophosphorylation of FAK at tyrosine 397 (Y397) subsequently induces additional phosphorylation at tyrosine residues such as Y925, resulting in the complete activation of FAK.
12)13) FAK-Src signaling stimulates the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway involved in cell survival and apoptosis. In addition, the FAT domain also interacts with the growth-factor-receptor-binding protein 2 (GRB2) mediated by Y925 phosphorylation, thereby inducing the activation of the mitogen-activating protein kinase (MAPK) cascade.
14)15)
In the present study, we investigated the effects of a newly synthesized talin modulator on SMC proliferation and migration and the underlying mechanism. We further identified that oral administration of the talin modulator inhibited neointimal formation after femoral arterial injury in apolipoprotein E knockout (ApoE KO) mice.
Go to :

METHODS
Synthesis of a talin modulator
A mixture of 2-chloroacetamide, N,N-dimethylformamide, K2CO3, and 2-methoxyphenol was stirred overnight. The residue was diluted with ethyl acetate and dried with anhydrous MgSO4. After separation using SiO2 column chromatography with ethyl acetate/n-hexane (1:1), the compound (0.2207 mM) and oxalyl chloride (0.509 mM) in dichloroethane were stirred at 85°C for 5 hours and concentrated under reduced pressure. Then, 4-bromo-3-methoxyaniline (0.221 mM) and methanol were added and stirred for 30 minutes at 0°C, and then overnight at room temperature (RT). The mixture was dried over MgSO4 and concentrated under reduced pressure. The final compound (N-((4-bromo-3-methoxyphenyl)carbamoyl)-2-(2-methoxyphenoxy)acetamide; talin modulator) was subjected to SiO2 column chromatography with ethyl acetate/n-hexane (1:3); 1H-NMR (dimethyl sulfoxide [DMSO], 500 MHz) δ 10.72 (s, 1H), 10.31 (s, 1H), 7.49 (d, 1H, J = 7.5Hz), 7.31 (s, 1H), 7.16 (d, 1H, J = 9.0Hz), 7.01 (d, 1H, J = 7.5Hz), 6.96–6.85 (m, 3H), 4.81 (s, 2H), 3.82 (s, 3H), 3.78 (s, 3H); LC-MS ([M+Na]+431), and was finally dissolved in DMSO (D8418, Sigma-Aldrich, St. Louis, MO, USA).
Surface plasmon resonance
Surface plasmon resonance (SPR) was performed to confirm the interaction between the talin modulator and talin protein as previously reported.
16) Briefly, the response curves were obtained using a ProteOn XPR36 system equipped with a sensor chip and ProteOn XPR36 control software ProteOn Manager v.3.1.0.6 (both from Bio-Rad, Hercules, CA, USA). Serially diluted samples were injected through the channels of the HTE chip for 1 minute at 100 μL/min, and stopped followed by injection with phosphate buffered saline (PBS) containing 0.005% Tween-20 to obtain the dissociation curves. The response unit of the empty channel was used for normalization.
Cell culture
Human aortic SMCs (HAoSMCs; CC-2571) were cultured in SMC growth medium-2 (CC-3182, both from Lonza, Basel, Switzerland) supplemented with 100 U/mL penicillin/streptomycin (#15140, Gibco) at 37°C in a 5% CO2 humidified incubator. After 24 hours of attachement, HAoSMCs were treated with the talin modulator or with an equivalent amount of DMSO (vehicle control). All phase-contrast images were obtained using an upright microscope (DMI 300B, Leica Microsystems, Wetzlar, Germany).
Cell proliferation assay
Cell proliferation was assessed by water-soluble tetrazolium salt assay (WST-1 assay; 05015944001, Roche, Basel, Switzerland). HAoSMCs were plated and treated with varying concentrations of the talin modulator or equivalent volume of DMSO. After 24 hours, the cells were incubated with the WST-1 reagent for 2 hours, and absorbance at 450 nm and 655 nm (reference wavelength) was detected using a microplate reader (iMark, Bio-Rad).
Wound healing migration assay
HAoSMCs were plated in 6-well plates and allowed to grow to be confluent for 24 hours. A scratch wound was made across the midline of each well using a sterile pipette tip and washed with PBS. The cells were then treated with the talin modulator or DMSO, and phase-contrast images of the wounded area were acquired at 0, 8, 12, and 24 hours after scratching and treatment. The area covered by migrated cells across the wounded line was quantified using Image J software (v1.32, National Institutes of Health, Bethesda, MD, USA).
Western blotting
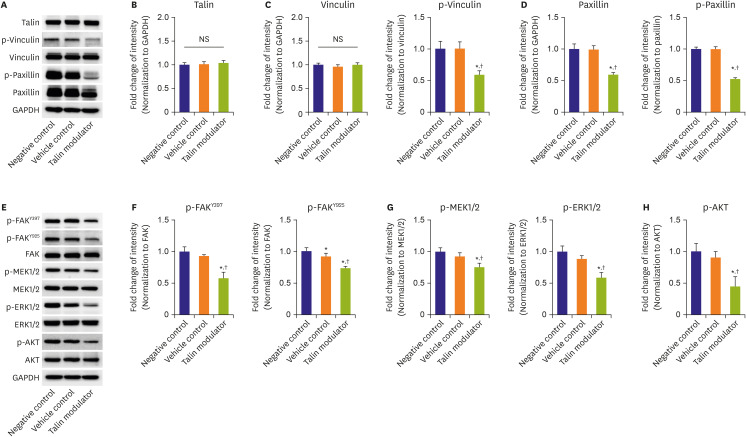
Total protein from HAoSMCs was extracted using cell lysis buffer (#9803, Cell Signaling Technology, Danvers, MA, USA). Equal amounts of the lysate were resolved on 8% or 10% gels by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (10600023, GE Healthcare, Chicago, IL, USA). The membranes were blocked with 5% skim milk in 0.1% tris-buffered saline and 0.1% Tween-20 for 1 hour at RT, and incubated with the following primary antibodies overnight at 4°C: talin (T3287), vinculin (V9131, both from Sigma-Aldrich), paxillin (#610051, BD Biosciences, San Jose, CA, USA), AKT (sc-1618, Santa Cruz, Dallas, TX, USA), p-vinculin (#44-1078G, Thermo Fisher Scientific, Waltham, MA, USA), p-paxillin (#2541), p-FAKY397 (#8556), p-FAKY925 (#3284), FAK (#13009), p-AKT (#9271), p-mitogen-activated protein kinase kinase 1/2 (MEK1/2; #9121), MEK1/2 (#9122), p-extracellular signal-regulated kinase 1/2 (ERK1/2; #9106), and ERK1/2 (#9102, all from Cell Signaling Technology). The membranes were washed and incubated with anti-mouse (#7076), anti-rabbit (#7074, both from Cell Signaling Technology), or anti-goat (HAF109, R&D systems, Minneapolis, MN, USA) HRP-conjugated secondary antibodies for 1 hour at RT. The protein bands were visualized by Clarity Western ECL Substrate (#1705061) and ChemiDoc Touch Imaging System (both from Bio-Rad). All band intensities were normalized to glyceraldehyde 3-phosphate dehydrogenase (G8795, Sigma-Aldrich) and analyzed using Quantity One software (Bio-Rad).
Immunofluorescence staining
HAoSMCs were fixed with 2% paraformaldehyde (PFA; P6148) and permeablized using 0.1% Triton X-100 (×100, both from Sigma-Aldrich). The cells were blocked with 5% normal goat serum (NGS; #16210, Gibco), and incubated with phosphohistone H3 (PHH3) antibody (06-570, Merck Millipore, Burlington, MA, USA) and Alexa Fluor 594-conjugated goat anti-rabbit immunoglobulin G (IgG) (A11012, Thermo Fisher Scientific). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (D9542, Sigma-Aldrich). Immunofluorescent images were acquired using a BX61 motorized system microscope (Olympus, Tokyo, Japan).
Induction of femoral arterial injury
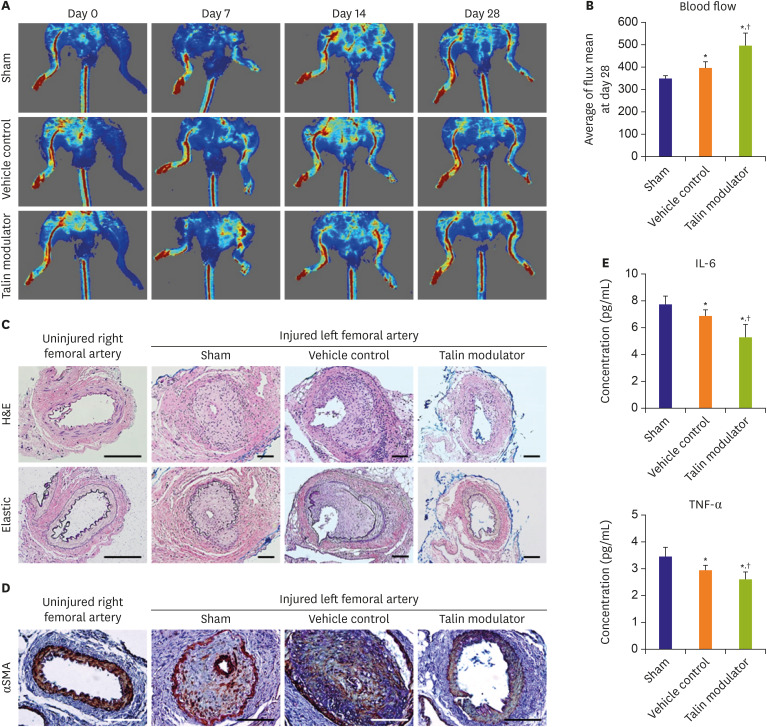
All animal experiments were approved by the Institutional Animal Care and Use Committee of Korea University College of Medicine (KOREA-2017-0126). Male, 10-week-old ApoE KO mice (#002052, The Jackson Laboratory, Bar Harbor, ME, USA) were anesthetized by intraperitoneal (IP) injection of ketamine (80 mg/kg; Yuhan, Seoul, Korea) and xylazine (8 mg/kg; Bayer, Leverkusen, Germany). After a groin incision, the left femoral arteries were injured by passing a sterile wire followed by ligation. ApoE KO mice were randomly assigned to the following 3 groups; sham (subjected to the procedure alone; n=5), vehicle control (DMSO; n=5), and talin modulator (5 mg/kg; n=6). The talin modulator and an equivalent volume of DMSO were prepared in 0.5% carboxymethylcellulose sodium salt (C5678, Sigma-Aldrich) for administration via oral gavage daily for 28 days. The mice were anesthetized at 7, 14, and 28 days post-procedure and the blood flow in the ventral side was analyzed using a laser Doppler imager (Moor Instruments, Devon, UK).
Histological analyses
At day 28, all mice were euthanized by IP injection of ketamine and xylazine, and femoral arteries were harvested. PFA-fixed paraffin-embedded arteries were sectioned in a 5 μm of thickness and stained with hematoxylin and eosin or elastic staining solutions.
After de-paraffinization, the tissue sections were incubated with proteinase K solution (#21627, Merck Millipore) for antigen retrieval and blocked with 5% NGS in 0.1% PBST. The sections were then incubated with α-smooth muscle actin (α-SMA; A2547, Sigma Aldrich) antibodies for 1 hour at RT and then with biotinylated anti-mouse IgG antibodies (BA-2000, Vector, Burlingame, CA, USA) for 30 minutes at RT. Subsequently, the sections were incubated with the ABC reagent (PK-6100) and peroxidase substrate (SK-4100), and mounted using VectaMount (H-5000, all from Vector). A BX61 motorized system microscope was used to obtain bright-field images.
Enzyme-linked immunosorbent assay
Blood serum collected from the abdominal vein of mice was separated using BD Vacutainer SST tubes (367955, BD Diagnostics, Franklin Lakes, NJ, USA). Mouse interleukin-6 (IL-6; M6000B) and tumor necrosis factor alpha (TNF-α; MTA00B, both from R&D Systems) enzyme-linked immunosorbent assay (ELISA) kits were used to evaluate serum levels after 28 days of femoral arterial injury and oral administration of the talin modulator. The ELISA reaction was measured using a microplate reader following the manufacturer's protocol.
Statistical analyses
Data are represented as the mean value±standard deviation. Significance of differences was analyzed by 1-way analysis of variance and Student-Newman Keuls test, and results were considered statistically significant when p<0.05. All experiments were repeated at least 3 times independently.
Go to :

DISCUSSION
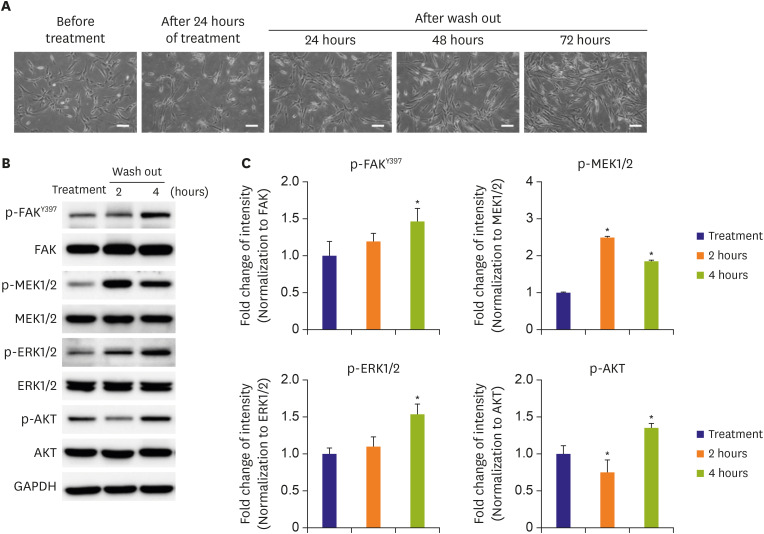
The present study demonstrated that a newly synthesized talin modulator exhibited anti-proliferative and anti-migratory effects in HAoSMCs, and its effects with respect to the regulation of downstream signaling pathways were reversible. Our study also revealed that oral administration of the talin modulator improved the blood flow and neointimal formation after femoral arterial injury in ApoE KO mice.
Talin has been implicated in linking integrin-mediated pathways related to cell proliferation and cell cycle progression. Talin-deficient epithelial cells were unable to recruit other focal adhesion proteins, and showed decreased incorporation of BrdU and 5-ethynyl-2′-deoxyuridine, and reduced PHH3 expression via modulating FAK phosphorylation.
9) The autophosphorylation of FAK at Y397 induces the phosphorylation of other tyrosine residues including Y925 which interacts with GRB2 of the MAPK pathway.
15) It has been reported that mutation of FAK at Y925 reduced cell proliferation, migration, and metastasis along with reduced activation of ERK1/2.
17) In addition, FAK has been found to be upstream of the PI3K/AKT signaling pathway involved in cell survival and apoptosis through its binding sites at Y397.
18) In accordance with previous reports, our findings demonstrated that significantly decreased phosphorylation of FAK at both Y397 and Y925 sites (
Figure 2E and F) due to treatment with the talin modulator resulted in subsequent downregulation of MAPK and PI3K signaling molecules (
Figure 2G and H). These downregulations resulted in a concentration-dependent decrease in cell proliferation and migration, and lower expression of PHH3 in talin modulator-treated cells (
Figure 1C-H).
Several studies have focused on inhibiting neointimal formation through suppressing the signaling pathways involved in SMC proliferation and migration.
3)19)20) In particular, Son et al.
21) reported that inhibition of FAK decreased SMC migration and neointimal thickening after balloon injury by reducing the phosphorylation of MAPKs and AKT. Similarly, the present study showed that administration of the talin modulator significantly suppressed neointimal formation and SMC expression within the neointima after arterial injury (
Figure 4C and D), via downregulating signaling pathways related to cell proliferation and migration (
Figure 2E).
A previous study had revealed the inhibitory effects of DMSO on SMC proliferation by suppressing DNA synthesis.
22) It has also been reported that 0.5% DMSO treatment showed a partial inhibition of SMC proliferation. In our study, DMSO was used as a solvent for the talin modulator and treatment with 0.5% DMSO as vehicle control partially, but not significantly affected cell proliferation relative to that in the negative control (
Figure 1E and F,
Figure 2G). Moreover, there were significant differences in the blood flow of injured femoral arteries and in the proinflammatory cytokine levels after administration of DMSO (vehicle control) relative to that in the sham group (
Figure 4B and E). These observations are supported by a previous study which reported that injection of 40% DMSO prevented the decrease in the blood flow and thrombotic occlusion in mouse carotid arteries after injury.
23)
In summary, this study showed that the talin modulator could attenuate neointimal formation after femoral arterial injury. The inhibitory effects of the talin modulator are mediated through suppression of SMC proliferation and migration via modulation of focal adhesion and downstream signaling molecules of talin. Based on our study, the talin modulator may be an effective anti-atherosclerotic drug by suppressing neointimal hyperplasia for the treatment of cardiovascular diseases.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download