INTRODUCTION
MEASUREMENT OF SALT INTAKE
Table 1
Studies that evaluated the validity of spot urine collection method for the estimation of sodium intake
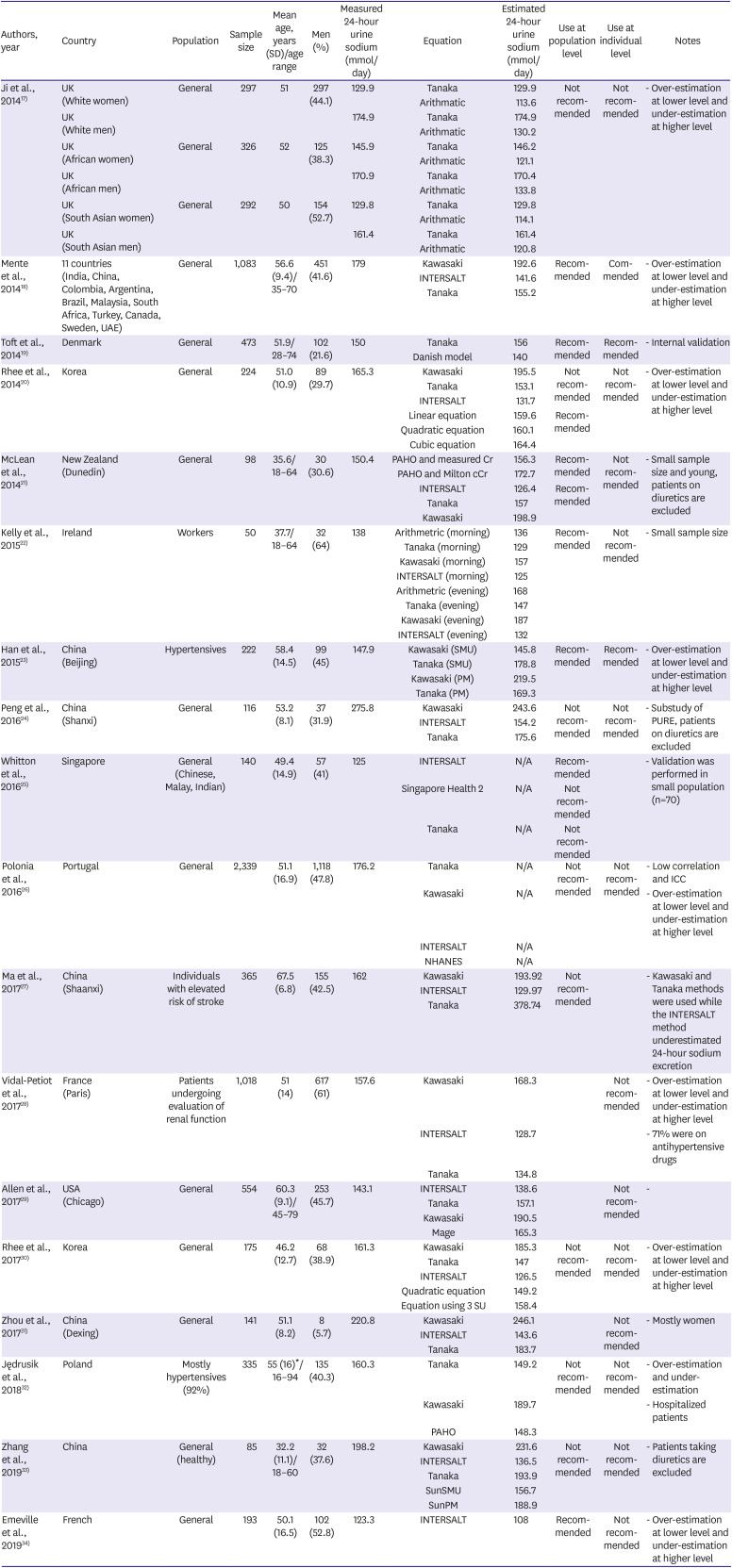
| Authors, year | Country | Population | Sample size | Mean age, years (SD)/age range | Men (%) | Measured 24-hour urine sodium (mmol/day) | Equation | Estimated 24-hour urine sodium (mmol/day) | Use at population level | Use at individual level | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ji et al., 201417) | UK (White women) | General | 297 | 51 | 297 (44.1) | 129.9 | Tanaka | 129.9 | Not recommended | Not recommended | - Over-estimation at lower level and under-estimation at higher level |
| Arithmatic | 113.6 | ||||||||||
| UK (White men) | 174.9 | Tanaka | 174.9 | ||||||||
| Arithmatic | 130.2 | ||||||||||
| UK (African women) | General | 326 | 52 | 125 (38.3) | 145.9 | Tanaka | 146.2 | ||||
| Arithmatic | 121.1 | ||||||||||
| UK (African men) | 170.9 | Tanaka | 170.4 | ||||||||
| Arithmatic | 133.8 | ||||||||||
| UK (South Asian women) | General | 292 | 50 | 154 (52.7) | 129.8 | Tanaka | 129.8 | ||||
| Arithmatic | 114.1 | ||||||||||
| UK (South Asian men) | 161.4 | Tanaka | 161.4 | ||||||||
| Arithmatic | 120.8 | ||||||||||
| Mente et al., 201418) | 11 countries (India, China, Colombia, Argentina, Brazil, Malaysia, South Africa, Turkey, Canada, Sweden, UAE) | General | 1,083 | 56.6 (9.4)/35–70 | 451 (41.6) | 179 | Kawasaki | 192.6 | Recommended | Commended | - Over-estimation at lower level and under-estimation at higher level |
| INTERSALT | 141.6 | ||||||||||
| Tanaka | 155.2 | ||||||||||
| Toft et al., 201419) | Denmark | General | 473 | 51.9/28–74 | 102 (21.6) | 150 | Tanaka | 156 | Recommended | Recommended | - Internal validation |
| Danish model | 140 | ||||||||||
| Rhee et al., 201420) | Korea | General | 224 | 51.0 (10.9) | 89 (29.7) | 165.3 | Kawasaki | 195.5 | Not recommended | Not recommended | - Over-estimation at lower level and under-estimation at higher level |
| Tanaka | 153.1 | ||||||||||
| INTERSALT | 131.7 | ||||||||||
| Linear equation | 159.6 | Recommended | |||||||||
| Quadratic equation | 160.1 | ||||||||||
| Cubic equation | 164.4 | ||||||||||
| McLean et al., 201421) | New Zealand (Dunedin) | General | 98 | 35.6/18–64 | 30 (30.6) | 150.4 | PAHO and measured Cr | 156.3 | Recommended | Not recommended | - Small sample size and young, patients on diuretics are excluded |
| PAHO and Milton cCr | 172.7 | ||||||||||
| INTERSALT | 126.4 | Recommended | |||||||||
| Tanaka | 157 | ||||||||||
| Kawasaki | 198.9 | ||||||||||
| Kelly et al., 201522) | Ireland | Workers | 50 | 37.7/18–64 | 32 (64) | 138 | Arithmetric (morning) | 136 | Recommended | Not recommended | - Small sample size |
| Tanaka (morning) | 129 | ||||||||||
| Kawasaki (morning) | 157 | ||||||||||
| INTERSALT (morning) | 125 | ||||||||||
| Arithmetric (evening) | 168 | ||||||||||
| Tanaka (evening) | 147 | ||||||||||
| Kawasaki (evening) | 187 | ||||||||||
| INTERSALT (evening) | 132 | ||||||||||
| Han et al., 201523) | China (Beijing) | Hypertensives | 222 | 58.4 (14.5) | 99 (45) | 147.9 | Kawasaki (SMU) | 145.8 | Recommended | Recommended | - Over-estimation at lower level and under-estimation at higher level |
| Tanaka (SMU) | 178.8 | ||||||||||
| Kawasaki (PM) | 219.5 | ||||||||||
| Tanaka (PM) | 169.3 | ||||||||||
| Peng et al., 201624) | China (Shanxi) | General | 116 | 53.2 (8.1) | 37 (31.9) | 275.8 | Kawasaki | 243.6 | Not recommended | Not recommended | - Substudy of PURE, patients on diuretics are excluded |
| INTERSALT | 154.2 | ||||||||||
| Tanaka | 175.6 | ||||||||||
| Whitton et al., 201625) | Singapore | General (Chinese, Malay, Indian) | 140 | 49.4 (14.9) | 57 (41) | 125 | INTERSALT | N/A | Recommended | - Validation was performed in small population (n=70) | |
| Singapore Health 2 | N/A | Not recommended | |||||||||
| Tanaka | N/A | Not recommended | |||||||||
| Polonia et al., 201626) | Portugal | General | 2,339 | 51.1 (16.9) | 1,118 (47.8) | 176.2 | Tanaka | N/A | Not recommended | Not recommended | - Low correlation and ICC |
| Kawasaki | N/A | - Over-estimation at lower level and under-estimation at higher level | |||||||||
| INTERSALT | N/A | ||||||||||
| NHANES | N/A | ||||||||||
| Ma et al., 201727) | China (Shaanxi) | Individuals with elevated risk of stroke | 365 | 67.5 (6.8) | 155 (42.5) | 162 | Kawasaki | 193.92 | Not recommended | - Kawasaki and Tanaka methods were used while the INTERSALT method underestimated 24-hour sodium excretion | |
| INTERSALT | 129.97 | ||||||||||
| Tanaka | 378.74 | ||||||||||
| Vidal-Petiot et al., 201728) | France (Paris) | Patients undergoing evaluation of renal function | 1,018 | 51 (14) | 617 (61) | 157.6 | Kawasaki | 168.3 | Not recommended | - Over-estimation at lower level and under-estimation at higher level | |
| INTERSALT | 128.7 | - 71% were on antihypertensive drugs | |||||||||
| Tanaka | 134.8 | ||||||||||
| Allen et al., 201729) | USA (Chicago) | General | 554 | 60.3 (9.1)/45–79 | 253 (45.7) | 143.1 | INTERSALT | 138.6 | Not recommended | - | |
| Tanaka | 157.1 | ||||||||||
| Kawasaki | 190.5 | ||||||||||
| Mage | 165.3 | ||||||||||
| Rhee et al., 201730) | Korea | General | 175 | 46.2 (12.7) | 68 (38.9) | 161.3 | Kawasaki | 185.3 | Not recommended | Not recommended | - Over-estimation at lower level and under-estimation at higher level |
| Tanaka | 147 | ||||||||||
| INTERSALT | 126.5 | ||||||||||
| Quadratic equation | 149.2 | ||||||||||
| Equation using 3 SU | 158.4 | ||||||||||
| Zhou et al., 201731) | China (Dexing) | General | 141 | 51.1 (8.2) | 8 (5.7) | 220.8 | Kawasaki | 246.1 | Not recommended | - Mostly women | |
| INTERSALT | 143.6 | ||||||||||
| Tanaka | 183.7 | ||||||||||
| Jędrusik et al., 201832) | Poland | Mostly hypertensives (92%) | 335 | 55 (16)*/16–94 | 135 (40.3) | 160.3 | Tanaka | 149.2 | Not recommended | Not recommended | - Over-estimation and under-estimation |
| Kawasaki | 189.7 | - Hospitalized patients | |||||||||
| PAHO | 148.3 | ||||||||||
| Zhang et al., 201933) | China | General (healthy) | 85 | 32.2 (11.1)/18–60 | 32 (37.6) | 198.2 | Kawasaki | 231.6 | Not recommended | Not recommended | - Patients taking diuretics are excluded |
| INTERSALT | 136.5 | ||||||||||
| Tanaka | 193.9 | ||||||||||
| SunSMU | 156.7 | ||||||||||
| SunPM | 188.9 | ||||||||||
| Emeville et al., 201934) | French | General | 193 | 50.1 (16.5) | 102 (52.8) | 123.3 | INTERSALT | 108 | Recommended | Not recommended | - Over-estimation at lower level and under-estimation at higher level |
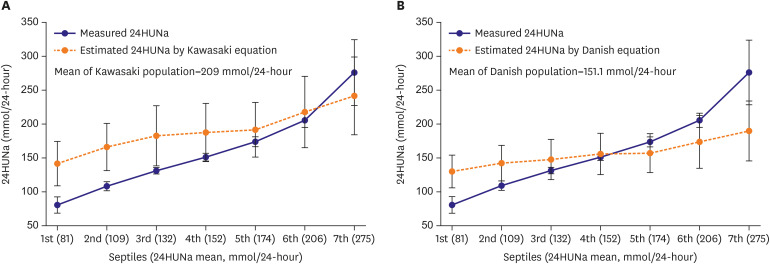 | Figure 1Comparison between measured and estimated 24HUNa in septile groups divided according to measured 24HUNa. Estimated 24-hour urine sodium was calculated by using previously suggested equations. (A) Kawasaki, and (B) Danish equation. When the 24HUNa is calculated by applying the formulas to groups with smaller 24HUNa than that of formula development population, there is a tendency of overestimation of population mean 24-hour urine sodium. On the other hands, in groups with greater 24-hour urine sodium than that of the formula development population, there is a tendency of underestimation of 24-hour urine sodium.24HUNa = 24-hour urine sodium excretion.
|
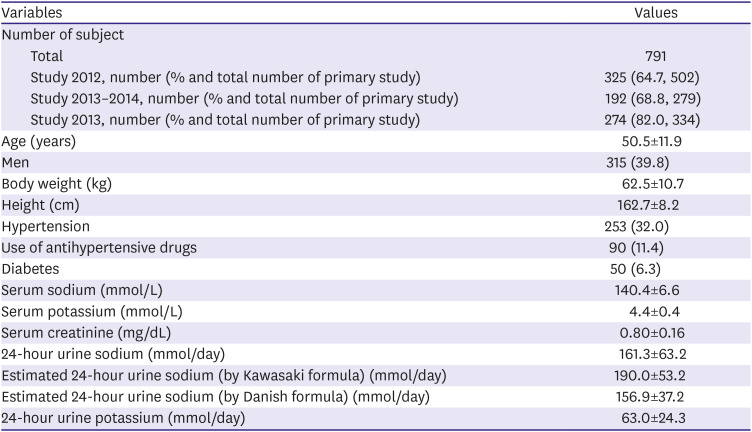
Table 2
Demographic and clinical characteristic of study population
SODIUM INTAKE AND BLOOD PRESSURE
Table 3
Cross-sectional epidemiologic studies that evaluated the association between sodium intake and blood pressure
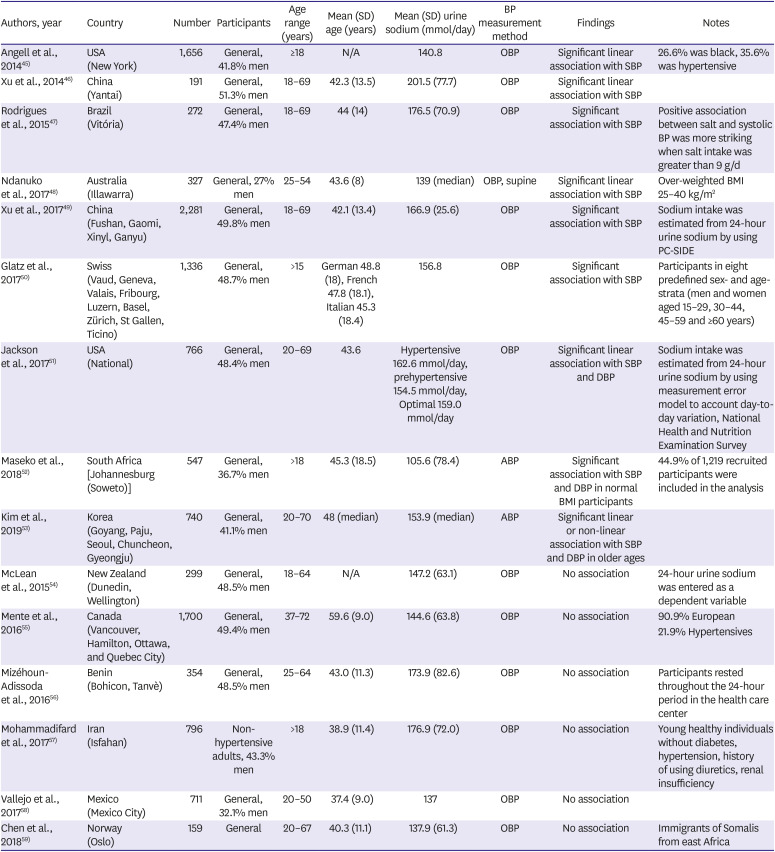
| Authors, year | Country | Number | Participants | Age range (years) | Mean (SD) age (years) | Mean (SD) urine sodium (mmol/day) | BP measurement method | Findings | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Angell et al., 201445) | USA (New York) | 1,656 | General, 41.8% men | ≥18 | N/A | 140.8 | OBP | Significant linear association with SBP | 26.6% was black, 35.6% was hypertensive |
| Xu et al., 201446) | China (Yantai) | 191 | General, 51.3% men | 18–69 | 42.3 (13.5) | 201.5 (77.7) | OBP | Significant linear association with SBP | |
| Rodrigues et al., 201547) | Brazil (Vitória) | 272 | General, 47.4% men | 18–69 | 44 (14) | 176.5 (70.9) | OBP | Significant association with SBP | Positive association between salt and systolic BP was more striking when salt intake was greater than 9 g/d |
| Ndanuko et al., 201748) | Australia (Illawarra) | 327 | General, 27% men | 25–54 | 43.6 (8) | 139 (median) | OBP, supine | Significant linear association with SBP | Over-weighted BMI 25–40 kg/m2 |
| Xu et al., 201749) | China (Fushan, Gaomi, Xinyl, Ganyu) | 2,281 | General, 49.8% men | 18–69 | 42.1 (13.4) | 166.9 (25.6) | OBP | Significant association with SBP | Sodium intake was estimated from 24-hour urine sodium by using PC-SIDE |
| Glatz et al., 201750) | Swiss (Vaud, Geneva, Valais, Fribourg, Luzern, Basel, Zürich, St Gallen, Ticino) | 1,336 | General, 48.7% men | >15 | German 48.8 (18), French 47.8 (18.1), Italian 45.3 (18.4) | 156.8 | OBP | Significant association with SBP | Participants in eight predefined sex- and age-strata (men and women aged 15–29, 30–44, 45–59 and ≥60 years) |
| Jackson et al., 201751) | USA (National) | 766 | General, 48.4% men | 20–69 | 43.6 | Hypertensive 162.6 mmol/day, prehypertensive 154.5 mmol/day, Optimal 159.0 mmol/day | OBP | Significant linear association with SBP and DBP | Sodium intake was estimated from 24-hour urine sodium by using measurement error model to account day-to-day variation, National Health and Nutrition Examination Survey |
| Maseko et al., 201852) | South Africa [Johannesburg (Soweto)] | 547 | General, 36.7% men | >18 | 45.3 (18.5) | 105.6 (78.4) | ABP | Significant association with SBP and DBP in normal BMI participants | 44.9% of 1,219 recruited participants were included in the analysis |
| Kim et al., 201953) | Korea (Goyang, Paju, Seoul, Chuncheon, Gyeongju) | 740 | General, 41.1% men | 20–70 | 48 (median) | 153.9 (median) | ABP | Significant linear or non-linear association with SBP and DBP in older ages | |
| McLean et al., 201554) | New Zealand (Dunedin, Wellington) | 299 | General, 48.5% men | 18–64 | N/A | 147.2 (63.1) | OBP | No association | 24-hour urine sodium was entered as a dependent variable |
| Mente et al., 201655) | Canada (Vancouver, Hamilton, Ottawa, and Quebec City) | 1,700 | General, 49.4% men | 37–72 | 59.6 (9.0) | 144.6 (63.8) | OBP | No association | 90.9% European |
| 21.9% Hypertensives | |||||||||
| Mizéhoun-Adissoda et al., 201656) | Benin (Bohicon, Tanvè) | 354 | General, 48.5% men | 25–64 | 43.0 (11.3) | 173.9 (82.6) | OBP | No association | Participants rested throughout the 24-hour period in the health care center |
| Mohammadifard et al., 201757) | Iran (Isfahan) | 796 | Non-hypertensive adults, 43.3% men | >18 | 38.9 (11.4) | 176.9 (72.0) | OBP | No association | Young healthy individuals without diabetes, hypertension, history of using diuretics, renal insufficiency |
| Vallejo et al., 201758) | Mexico (Mexico City) | 711 | General, 32.1% men | 20–50 | 37.4 (9.0) | 137 | OBP | No association | |
| Chen et al., 201859) | Norway (Oslo) | 159 | General | 20–67 | 40.3 (11.1) | 137.9 (61.3) | OBP | No association | Immigrants of Somalis from east Africa |
SODIUM INTAKE AND CARDIOVASCULAR DISEASE
Table 4
Cohort studies that evaluated the association between sodium intake and cardiovascular outcomes
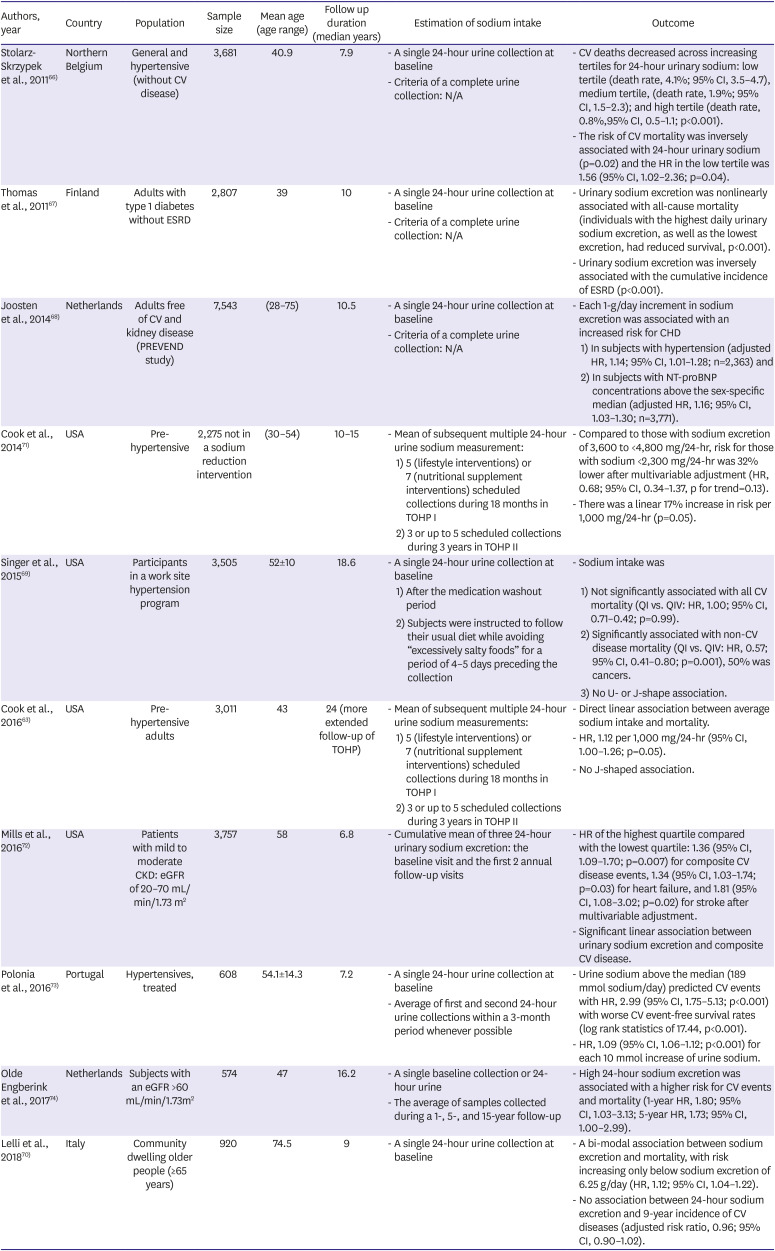
| Authors, year | Country | Population | Sample size | Mean age (age range) | Follow up duration (median years) | Estimation of sodium intake | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| Stolarz-Skrzypek et al., 201166) | Northern Belgium | General and hypertensive (without CV disease) | 3,681 | 40.9 | 7.9 | - A single 24-hour urine collection at baseline | - CV deaths decreased across increasing tertiles for 24-hour urinary sodium: low tertile (death rate, 4.1%; 95% CI, 3.5–4.7), medium tertile, (death rate, 1.9%; 95% CI, 1.5–2.3); and high tertile (death rate, 0.8%,95% CI, 0.5–1.1; p<0.001). | ||
| - Criteria of a complete urine collection: N/A | - The risk of CV mortality was inversely associated with 24-hour urinary sodium (p=0.02) and the HR in the low tertile was 1.56 (95% CI, 1.02–2.36; p=0.04). | ||||||||
| Thomas et al., 201167) | Finland | Adults with type 1 diabetes without ESRD | 2,807 | 39 | 10 | - A single 24-hour urine collection at baseline | - Urinary sodium excretion was nonlinearly associated with all-cause mortality (individuals with the highest daily urinary sodium excretion, as well as the lowest excretion, had reduced survival, p<0.001). | ||
| - Criteria of a complete urine collection: N/A | - Urinary sodium excretion was inversely associated with the cumulative incidence of ESRD (p<0.001). | ||||||||
| Joosten et al., 201468) | Netherlands | Adults free of CV and kidney disease (PREVEND study) | 7,543 | (28–75) | 10.5 | - A single 24-hour urine collection at baseline | - Each 1-g/day increment in sodium excretion was associated with an increased risk for CHD | ||
| - Criteria of a complete urine collection: N/A | 1) In subjects with hypertension (adjusted HR, 1.14; 95% CI, 1.01–1.28; n=2,363) and | ||||||||
| 2) In subjects with NT-proBNP concentrations above the sex-specific median (adjusted HR, 1.16; 95% CI, 1.03–1.30; n=3,771). | |||||||||
| Cook et al., 201471) | USA | Pre-hypertensive | 2,275 not in a sodium reduction intervention | (30–54) | 10–15 | - Mean of subsequent multiple 24-hour urine sodium measurement: | - Compared to those with sodium excretion of 3,600 to <4,800 mg/24-hr, risk for those with sodium <2,300 mg/24-hr was 32% lower after multivariable adjustment (HR, 0.68; 95% CI, 0.34–1.37, p for trend=0.13). | ||
| 1) 5 (lifestyle interventions) or 7 (nutritional supplement interventions) scheduled collections during 18 months in TOHP I | - There was a linear 17% increase in risk per 1,000 mg/24-hr (p=0.05). | ||||||||
| 2) 3 or up to 5 scheduled collections during 3 years in TOHP II | |||||||||
| Singer et al., 201569) | USA | Participants in a work site hypertension program | 3,505 | 52±10 | 18.6 | - A single 24-hour urine collection at baseline | - Sodium intake was | ||
| 1) After the medication washout period | 1) Not significantly associated with all CV mortality (QI vs. QIV: HR, 1.00; 95% CI, 0.71–0.42; p=0.99). | ||||||||
| 2) Subjects were instructed to follow their usual diet while avoiding “excessively salty foods” for a period of 4–5 days preceding the collection | 2) Significantly associated with non-CV disease mortality (QI vs. QIV: HR, 0.57; 95% CI, 0.41–0.80; p=0.001), 50% was cancers. | ||||||||
| 3) No U- or J-shape association. | |||||||||
| Cook et al., 201663) | USA | Pre-hypertensive adults | 3,011 | 43 | 24 (more extended follow-up of TOHP) | - Mean of subsequent multiple 24-hour urine sodium measurements: | - Direct linear association between average sodium intake and mortality. | ||
| 1) 5 (lifestyle interventions) or 7 (nutritional supplement interventions) scheduled collections during 18 months in TOHP I | - HR, 1.12 per 1,000 mg/24-hr (95% CI, 1.00–1.26; p=0.05). | ||||||||
| 2) 3 or up to 5 scheduled collections during 3 years in TOHP II | - No J-shaped association. | ||||||||
| Mills et al., 201672) | USA | Patients with mild to moderate CKD: eGFR of 20–70 mL/min/1.73 m2 | 3,757 | 58 | 6.8 | - Cumulative mean of three 24-hour urinary sodium excretion: the baseline visit and the first 2 annual follow-up visits | - HR of the highest quartile compared with the lowest quartile: 1.36 (95% CI, 1.09–1.70; p=0.007) for composite CV disease events, 1.34 (95% CI, 1.03–1.74; p=0.03) for heart failure, and 1.81 (95% CI, 1.08–3.02; p=0.02) for stroke after multivariable adjustment. | ||
| - Significant linear association between urinary sodium excretion and composite CV disease. | |||||||||
| Polonia et al., 201673) | Portugal | Hypertensives, treated | 608 | 54.1±14.3 | 7.2 | - A single 24-hour urine collection at baseline | - Urine sodium above the median (189 mmol sodium/day) predicted CV events with HR, 2.99 (95% CI, 1.75–5.13; p<0.001) with worse CV event-free survival rates (log rank statistics of 17.44, p<0.001). | ||
| - Average of first and second 24-hour urine collections within a 3-month period whenever possible | - HR, 1.09 (95% CI, 1.06–1.12; p<0.001) for each 10 mmol increase of urine sodium. | ||||||||
| Olde Engberink et al., 201774) | Netherlands | Subjects with an eGFR >60 mL/min/1.73m2 | 574 | 47 | 16.2 | - A single baseline collection or 24-hour urine | - High 24-hour sodium excretion was associated with a higher risk for CV events and mortality (1-year HR, 1.80; 95% CI, 1.03–3.13; 5-year HR, 1.73; 95% CI, 1.00–2.99). | ||
| - The average of samples collected during a 1-, 5-, and 15-year follow-up | |||||||||
| Lelli et al., 201870) | Italy | Community dwelling older people (≥65 years) | 920 | 74.5 | 9 | - A single 24-hour urine collection at baseline | - A bi-modal association between sodium excretion and mortality, with risk increasing only below sodium excretion of 6.25 g/day (HR, 1.12; 95% CI, 1.04–1.22). | ||
| - No association between 24-hour sodium excretion and 9-year incidence of CV diseases (adjusted risk ratio, 0.96; 95% CI, 0.90–1.02). | |||||||||
CONCLUSION
1) The spot urine collection method is inaccurate and should not be recommended to measure sodium intake at the individual level, and further studies for its use to measure sodium intake at the population level are required.
2) High sodium intake is positively associated with BP.
3) Associations between high sodium intake and CV outcomes are significant, but reverse causality cannot be ruled out.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download