1. Roberts R, Croft C, Gold HK, et al. Effect of propranolol on myocardial-infarct size in a randomized blinded multicenter trial. N Engl J Med. 1984; 311:218–225. PMID:
6377070.

2. Poirier L, Tobe SW. Contemporary use of β-blockers: clinical relevance of subclassification. Can J Cardiol. 2014; 30:S9–S15. PMID:
24684855.

3. Bloch Thomsen PE, Jons C, Raatikainen MJ, et al. Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: the Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study. Circulation. 2010; 122:1258–1264. PMID:
20837897.
4. Mak IT, Weglicki WB. Protection by beta-blocking agents against free radical-mediated sarcolemmal lipid peroxidation. Circ Res. 1988; 63:262–266. PMID:
2898307.

5. Dondo TB, Hall M, West RM, et al. β-blockers and mortality after acute myocardial infarction in patients without heart failure or ventricular dysfunction. J Am Coll Cardiol. 2017; 69:2710–2720. PMID:
28571635.

6. Yang JH, Hahn JY, Song YB, et al. Association of beta-blocker therapy at discharge with clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2014; 7:592–601. PMID:
24947717.
7. Kozma CM, Dickson M, Phillips AL, Meletiche DM. Medication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosis. Patient Prefer Adherence. 2013; 7:509–516. PMID:
23807840.

8. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011; 173:676–682. PMID:
21330339.

9. Hjalmarson A. Effects of beta blockade on sudden cardiac death during acute myocardial infarction and the postinfarction period. Am J Cardiol. 1997; 80:35J–9J.

10. von Arnim T. Medical treatment to reduce total ischemic burden: total ischemic burden bisoprolol study (TIBBS), a multicenter trial comparing bisoprolol and nifedipine. J Am Coll Cardiol. 1995; 25:231–238. PMID:
7798508.

11. Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981; 304:801–807. PMID:
7010157.
12. Beta-Blocker Heart Attack Trial Resarch Group. A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982; 247:1707–1714. PMID:
7038157.
13. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001; 357:1385–1390. PMID:
11356434.
14. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 130:e344–426. PMID:
25249585.

15. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 61:e78–140. PMID:
23256914.
16. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018; 39:119–177. PMID:
28886621.
17. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011; 58:2432–2446. PMID:
22055990.
18. Dahl Aarvik M, Sandven I, Dondo TB, et al. Effect of oral β-blocker treatment on mortality in contemporary post-myocardial infarction patients: a systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2019; 5:12–20. PMID:
30192930.

19. Park JJ, Kim SH, Kang SH, et al. Effect of β-blockers beyond 3 years after acute myocardial infarction. J Am Heart Assoc. 2018; 7:e007567. PMID:
29502101.
20. Puymirat E, Riant E, Aissaoui N, et al. β blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ. 2016; 354:i4801. PMID:
27650822.

21. Kimm H, Yun JE, Lee SH, Jang Y, Jee SH. Validity of the diagnosis of acute myocardial infarction in Korean national medical health insurance claims data: the Korean Heart Study (1). Korean Circ J. 2012; 42:10–15. PMID:
22363378.

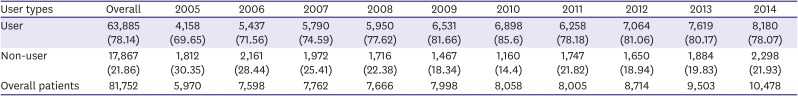
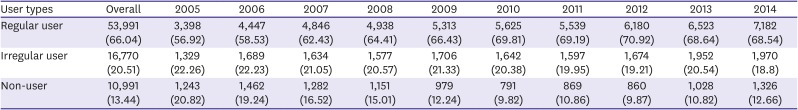
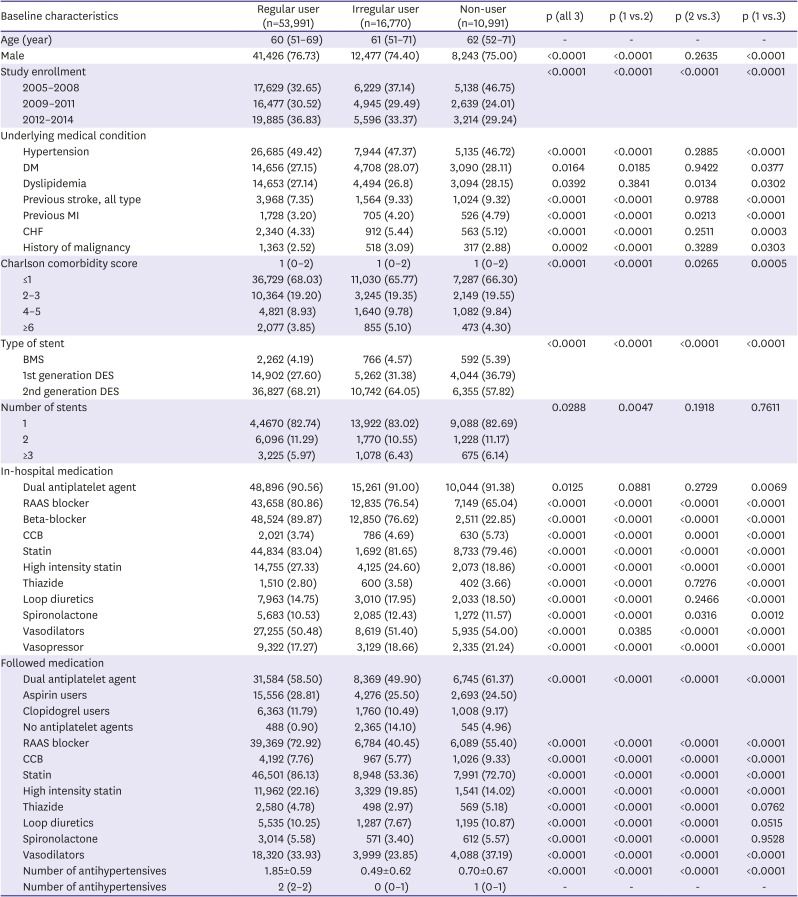
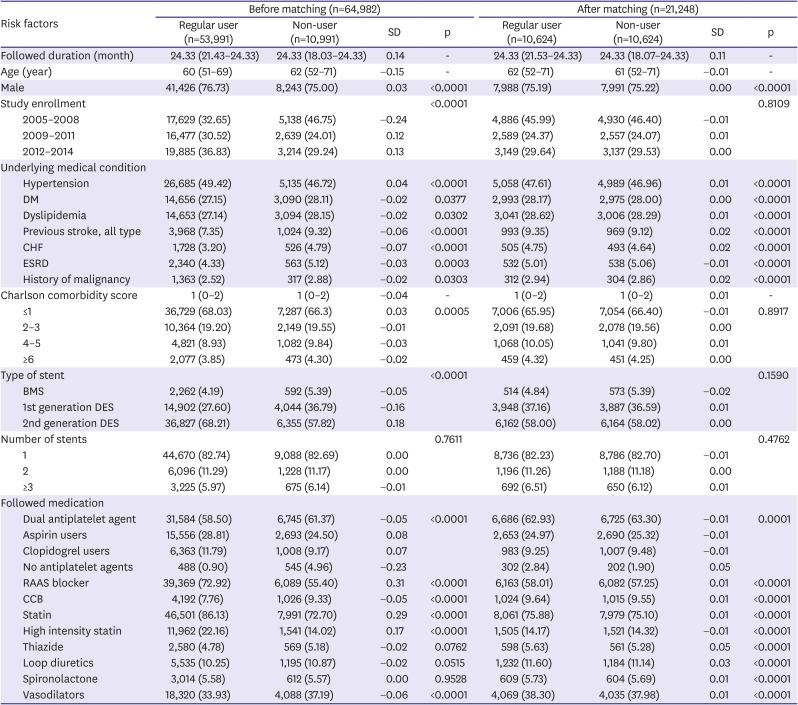




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download