IMR is a frequent complication, and it also has important clinical impacts on the outcomes of ischemic heart disease.
2,
3 However, its acute transient form has been reported infrequently. Moreover, using a left ventricular angiogram, Finelli et al.
4 demonstrated severe transient IMR in a patient with symptoms of recurrent heart failure. After introducing coronary revascularization technique, a few cases of resolution of acute IMR after coronary angioplasty have been reported.
5,
6 Although the pathogenesis of transient IMR is unclear, posteromedial papillary muscle dysfunction due to ischemia is suggested to be the main causative mechanism. Acute severe IMR in acute myocardial infarction is known as an irreversible mechanical complication caused by papillary muscle rupture and/or dysfunction, which is related to pap
50 illary muscle infarction. However, previous reports indicated that there is a transient form of IMR and that this IMR type could disappear after coronary angioplasty.
5,
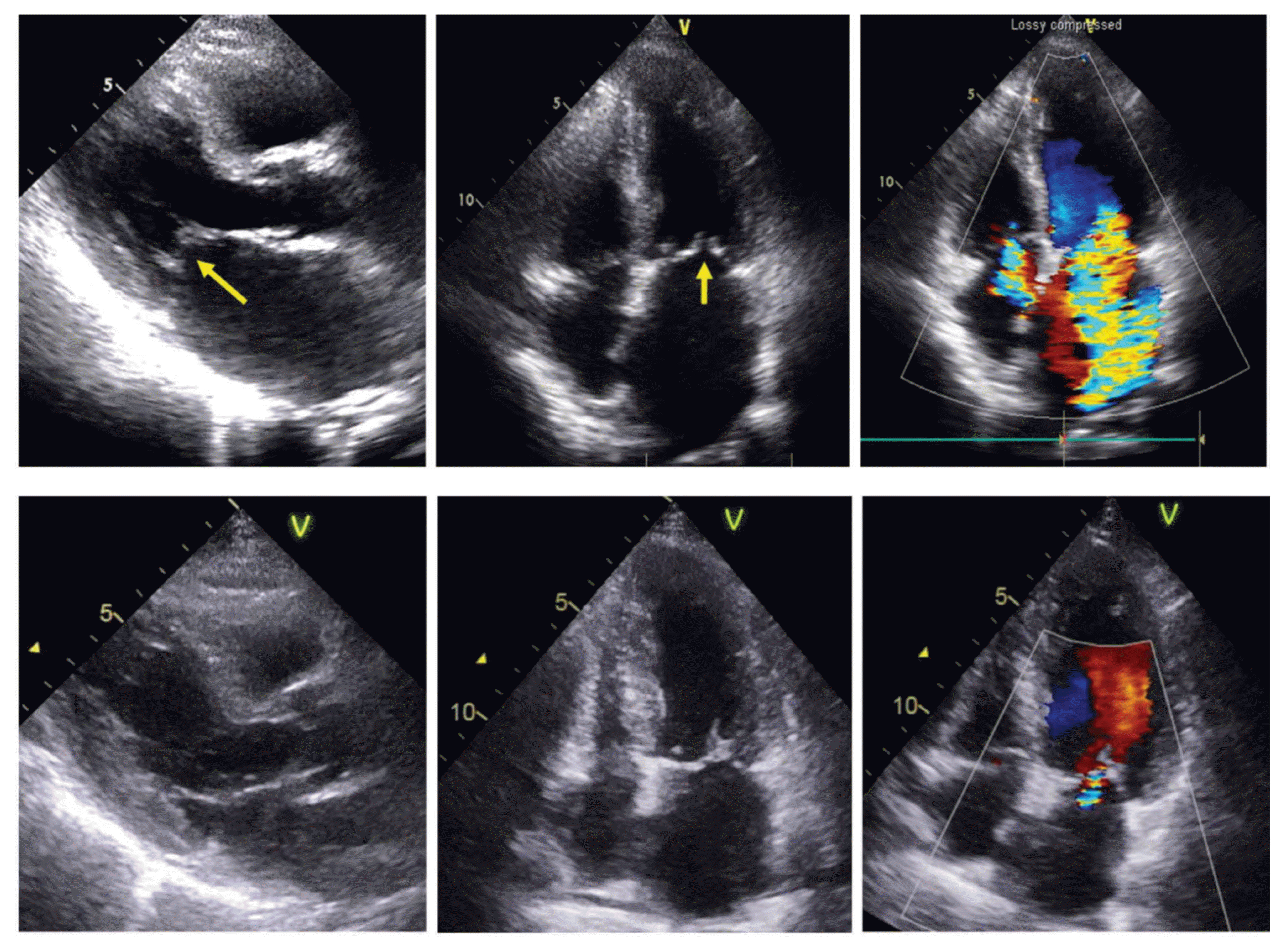
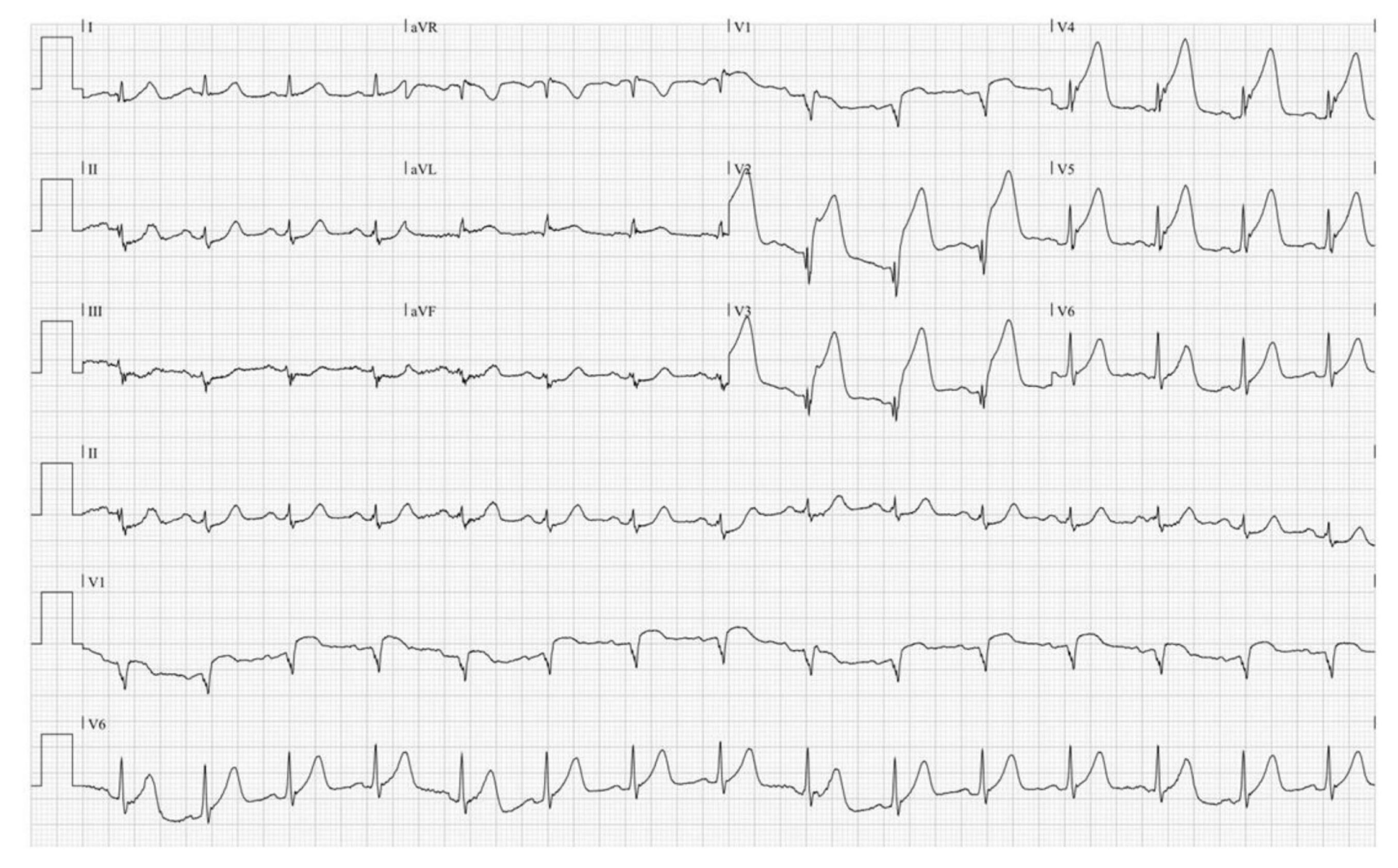
6 In our case, the patient with unstable angina who had minimal MR, acute severe MR and pulmonary edema were suddenly developed after treadmill exercise test. Remarkably, the acute severe IMR disappeared immediately after PCI for the RCA. To our knowledge, there is no echocardiographic documentation of abrupt IMR change in a patient with acute coronary syndrome. Recently, transient IMR due to coronary artery spasm was reported and the authors named it the “Eclipsed MR”.
1,
7,
8 Liang et al. suggested that the mechanism behind transient IMR due to spasms is global subendocardial ischemia, which results in apical displacement of both papillary muscles.
1 They also explained that the ejection fraction appeared to be preserved because of an acute decrease in afterload caused by severe acute MR. The feature of TTE in our patient was similar to that in global subendocardial ischemia, although the main cause, which is localized posteromedial papillary muscle dysfunction related to RCA stenosis, is different. Therefore, coronary artery disease should be considered in the differential diagnosis of patients with transient IMR even in situations where the mitral valve is structurally normal, wall motion abnormality is uncertain, and ejection fraction is preserved. The treatment of IMR remains inconclusive. In a population of patients with acute myocardial infarction, successful primary PCI was helpful in the improvement of IMR.
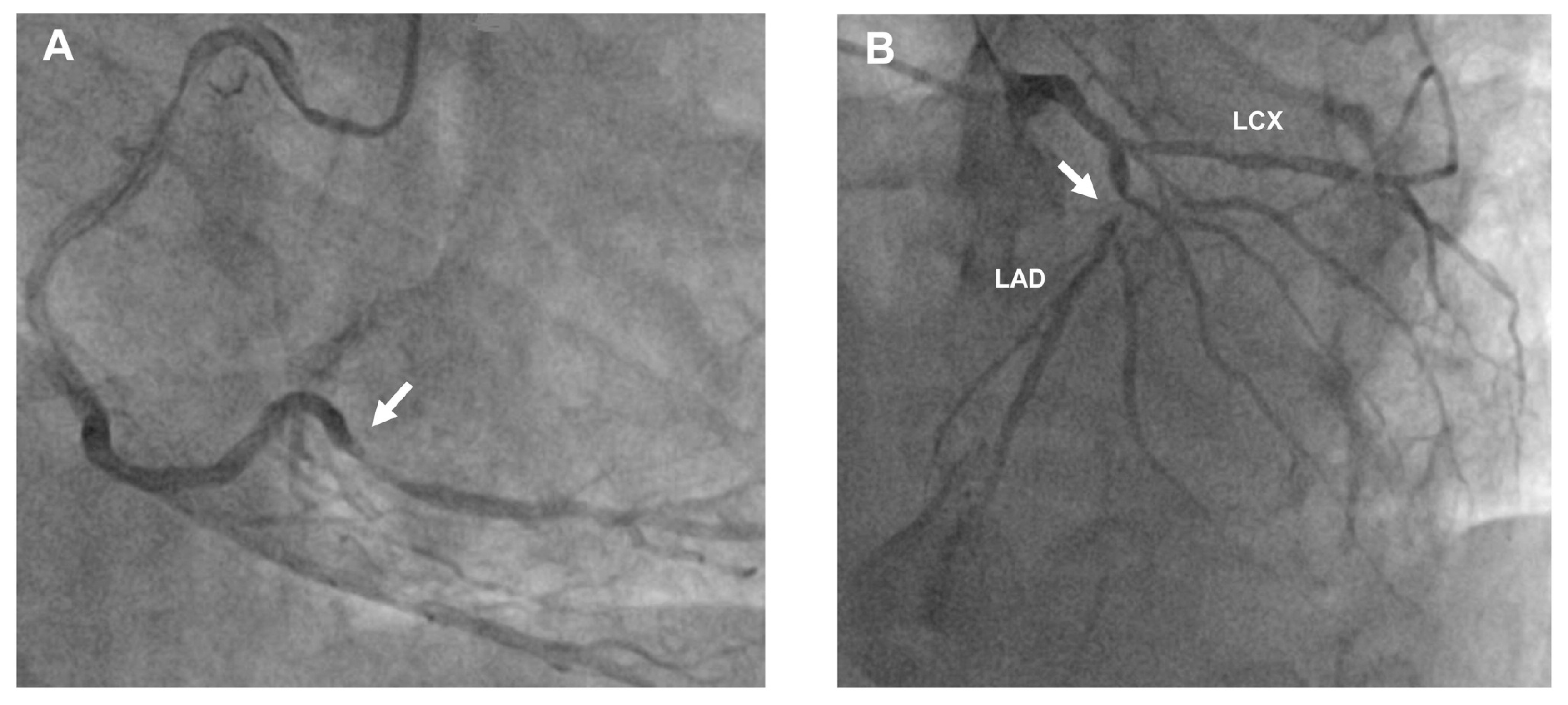
2,
3 In the current case, significant RCA stenosis was recorded in the coronary angiogram for acute anterior ST-elevated myocardial infarction. During the second episode of unstable angina, severe IMR was detected. Because we considered the possibility of papillary muscle dysfunction due to RCA stenosis, early revascularization could be decided. In conclusion, acute transient IMR is an infrequently reported and under-recognized complication. We described a case of acute transient IMR caused by acute coronary syndrome, in which the patient fully recovered immediately after PCI. We suggest that coronary artery disease should be considered in the differential diagnosis of patients with transient IMR. Early diagnosis and prompt therapeutic intervention may help improve the prognosis of these patients.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download