1. Agarwal CA, Mendenhall SD 3rd, Foreman KB, Owsley JQ. The course of the frontal branch of the facial nerve in relation to fascial planes: an anatomic study. Plast Reconstr Surg. 2010; Feb. 125(2):532–7.

2. Alkhalili KA, Hannallah JR, Alshyal GH, Nageeb MM, Abdel Aziz KM. The minipterional approach for ruptured and unruptured anterior circulation aneurysms: Our initial experience. Asian J Neurosurg. 2017; Jul–Sep. 12(3):466–74.

3. Ammirati M, Spallone A, Ma J, Cheatham M, Becker D. An anatomicosurgical study of the temporal branch of the facial nerve. Neurosurgery. 1993; Dec. 33(6):1038–43. discussion 44.

4. Aydin IH, Kadioglu HH, Tuzun Y, Kayaoglu CR, Takci E, Ozturk M. Postoperative anosmia after anterior communicating artery aneurysms surgery by the pterional approach. Minim Invasive Neurosurg. 1996; Sep. 39(3):71–3.

5. Babakurban ST, Cakmak O, Kendir S, Elhan A, Quatela VC. Temporal branch of the facial nerve and its relationship to fascial layers. Arch Facial Plast Surg. 2010; Jan–Feb. 12(1):16–23.

6. Caplan JM, Papadimitriou K, Yang W, Colby GP, Coon AL, Olivi A, et al. The minipterional craniotomy for anterior circulation aneurysms: initial experience with 72 patients. Neurosurgery. 2014; Jun. 10(Suppl 2):200–6. discussion 206–7.

7. Davies JM, Lawton MT. Advances in open microsurgery for cerebral aneurysms. Neurosurgery. 2014; Feb. 74(Suppl 1):S7–16.

8. Dzhindzhikhadze RS, Dreval ON, Lazarev VA, Kambiev RL. Minipterional craniotomy in surgery for anterior circle of Willis aneurysms. Zh Vopr Neirokhir Im N N Burdenko. 2016; 80(6):–58. –65.

9. Figueiredo EG, Deshmukh P, Nakaji P, Crusius MU, Crawford N, Spetzler RF, et al. The minipterional craniotomy: technical description and anatomic assessment. Neurosurgery. 2007; Nov. 61(5 Suppl 2):256–64. discussion 64–5.

10. Figueiredo EG, Deshmukh V, Nakaji P, Deshmukh P, Crusius MU, Crawford N, et al. An anatomical evaluation of the mini-supraorbital approach and comparison with standard craniotomies. Neurosurgery. 2006; Oct. 59(4 Suppl 2):ONS212-20. discussion ONS20.

11. Figueiredo EG, Welling LC, Preul MC, Sakaya GR, Neville I, Spetzler RF, et al. Surgical experience of minipterional craniotomy with 102 ruptured and unruptured anterior circulation aneurysms. J Clin Neurosci. 2016; May. 27:34–9.

12. Fischer G, Stadie A, Reisch R, Hopf NJ, Fries G, Bocher-Schwarz H, et al. The keyhole concept in aneurysm surgery: results of the past 20 years. Neurosurgery. 2011; Mar. 68(1 Suppl Operative):45–51. discussion 51.

13. Hernesniemi J, Ishii K, Niemela M, Smrcka M, Kivipelto L, Fujiki M, et al. Lateral supraorbital approach as an alternative to the classical pterional approach. Acta Neurochir Suppl. 2005; 94:17–21.

14. Kadri PA, Al-Mefty O. The anatomical basis for surgical preservation of temporal muscle. J Neurosurg. 2004; Mar. 100(3):517–22.

15. Mocco J, Komotar RJ, Raper DM, Kellner CP, Connolly ES, Solomon RA. The modified pterional keyhole craniotomy for open cerebrovascular surgery: a new workhorse? J Neurol Surg A Cent Eur Neurosurg. 2013; Nov. 74(6):400–4.

16. Mori K, Esaki T, Yamamoto T, Nakao Y. Individualized pterional keyhole clipping surgery based on a preoperative three-dimensional virtual osteotomy technique for unruptured middle cerebral artery aneurysm. Minim Invasive Neurosurg. 2011; Oct. 54(5–6):207–13.

17. Mori K, Wada K, Otani N, Tomiyama A, Toyooka T, Takeuchi S, et al. Keyhole strategy aiming at minimizing hospital stay for surgical clipping of unruptured middle cerebral artery aneurysms. J Neurosurg. 2018; Apr. 1. 1–8.

18. Park J. Supraorbital Keyhole Approach for Intracranial Aneurysms: Transitioning from Concerns to Confidence. J Korean Neurosurg Soc. 2020; Jan. 63(1):4–13.
19. Poblete T, Jiang X, Komune N, Matsushima K, Rhoton AL Jr. Preservation of the nerves to the frontalis muscle during pterional craniotomy. J Neurosurg. 2015; Jun. 122(6):1274–82.

20. Reisch R, Marcus HJ, Hugelshofer M, Koechlin NO, Stadie A, Kockro RA. Patients’ cosmetic satisfaction, pain, and functional outcomes after supraorbital craniotomy through an eyebrow incision. J Neurosurg. 2014; Sep. 121(3):730–4.

21. Rychen J, Croci D, Roethlisberger M, Nossek E, Potts M, Radovanovic I, et al. Minimally invasive alternative approaches to pterional craniotomy: a systematic review of the literature. World Neurosurg. 2018; May. 113:163–79.

22. Sturiale CL, La Rocca G, Puca A, Fernandez E, Visocchi M, Marchese E, et al. Minipterional craniotomy for treatment of unruptured middle cerebral artery aneurysms. A single-center comparative analysis with standard pterional approach as regard to safety and efficacy of aneurysm clipping and the advantages of reconstruction. Acta Neurochir Suppl. 2017; 124:93–100.

23. Tayebi Meybodi A, Lawton MT, Yousef S, Sanchez JJG, Benet A. Preserving the facial nerve during orbitozygomatic craniotomy: surgical anatomy assessment and stepwise illustration. World Neurosurg. 2017; Sep. 105:359–68.

24. Toyooka T, Wada K, Otani N, Tomiyama A, Takeuchi S, Tomura S, et al. Potential risks and limited indications of the supraorbital keyhole approach for clipping internal carotid artery Aneurysms. World Neurosurg X. 2019; Feb. 2:100025.

25. Tra H, Huynh T, Nguyen B. Minipterional and supraorbital keyhole craniotomies for ruptured anterior circulation aneurysms: experience at single center. World Neurosurg. 2018; Jan. 109:36–9.

26. Vaca EE, Purnell CA, Gosain AK, Alghoul MS. Postoperative temporal hollowing: Is there a surgical approach that prevents this complication? A systematic review and anatomic illustration. J Plast Reconstr Aesthet Surg. 2017; Mar. 70(3):401–15.

27. Welling LC, Figueiredo EG, Wen HT, Gomes MQ, Bor-Seng-Shu E, Casarolli C, et al. Prospective randomized study comparing clinical, functional, and aesthetic results of minipterional and classic pterional craniotomies. J Neurosurg. 2015; May. 122(5):1012–9.

28. Wong JH, Tymianski R, Radovanovic I, Tymianski M. Minimally invasive microsurgery for cerebral aneurysms. Stroke. 2015; Sep. 46(9):2699–706.

29. Yasargil MG, Fox JL. The microsurgical approach to intracranial aneurysms. Surg Neurol. 1975; Jan. 3(1):7–14.
30. Yasargil MG, Reichman MV, Kubik S. Preservation of the frontotemporal branch of the facial nerve using the interfascial temporalis flap for pterional craniotomy. Technical article. J Neurosurg. 1987; Sep. 67(3):463–6.




 PDF
PDF Citation
Citation Print
Print



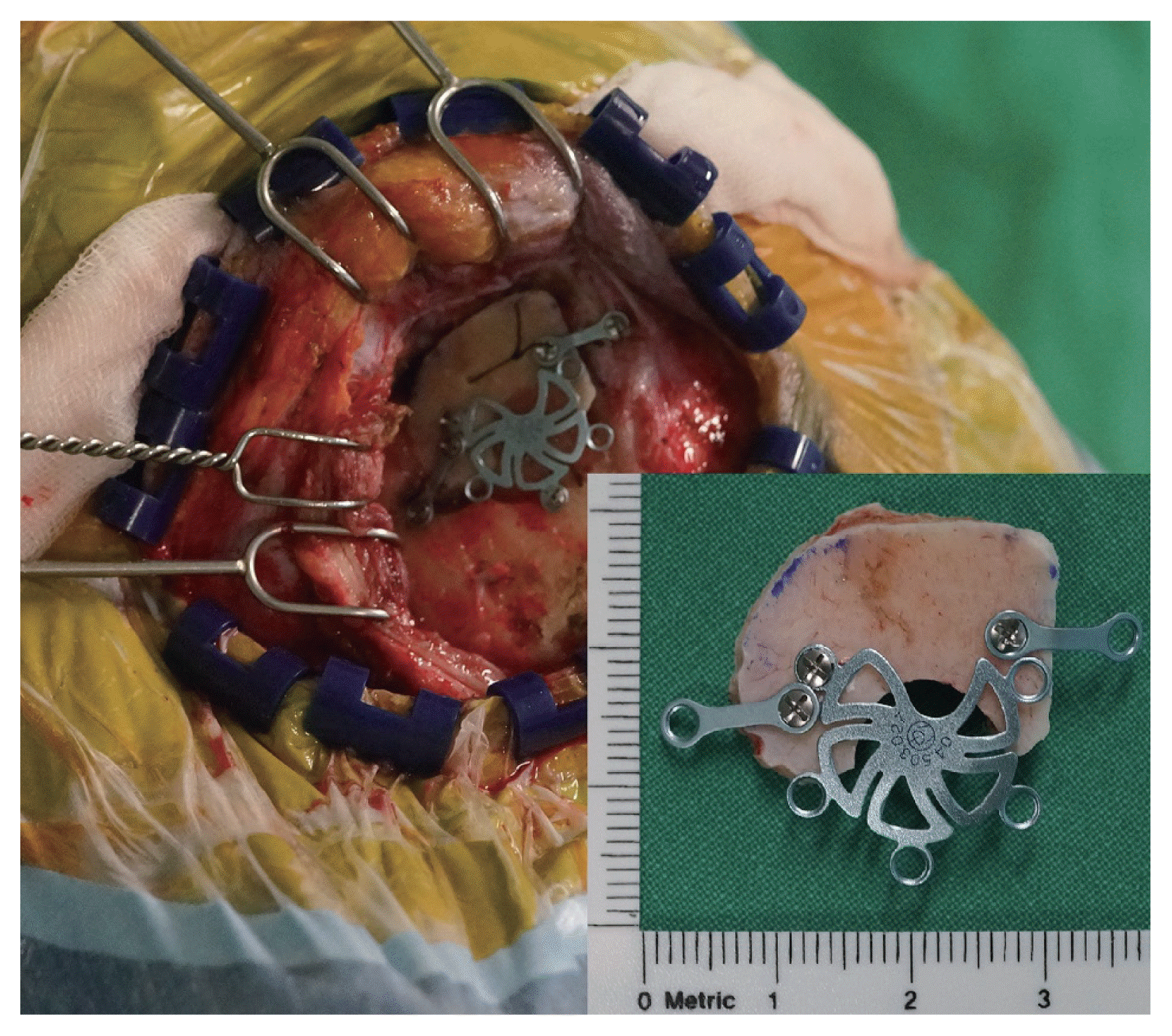
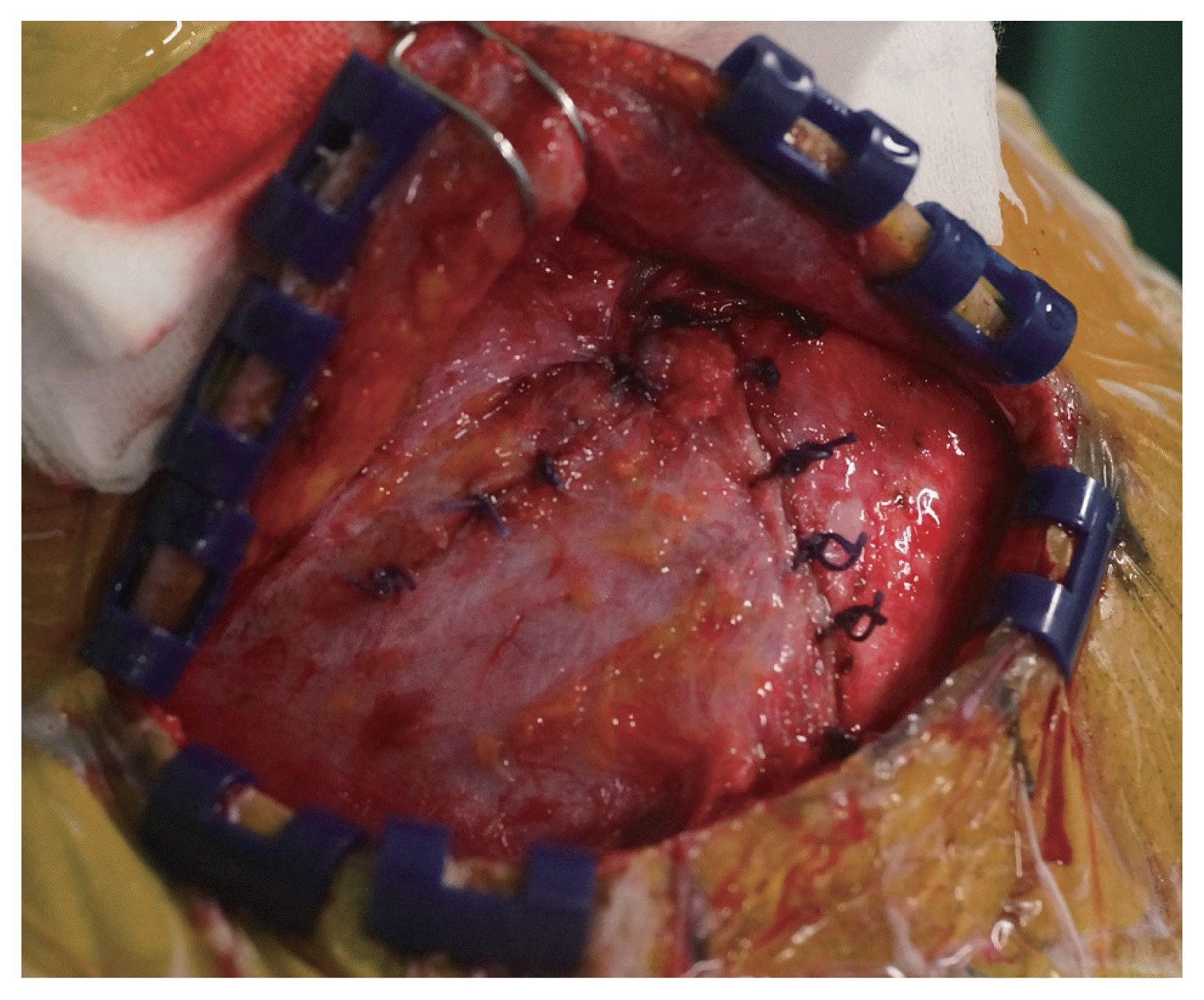
 XML Download
XML Download