Abstract
Intra-arterial embolization of juvenile nasopharyngeal angiofibroma (JNA) prior to surgical resection is the preferred approach to minimize blood loss during surgical resection of the tumor. However, the presence of external carotid artery–internal carotid artery (ECA-ICA) anastomoses may hinder complete tumor embolization due to the associated risk for embolic complications. Here, we evaluate the use of a balloon-assisted embolization (BAE) technique in the treatment of JNA. We conducted a retrospective review of JNA patients who underwent tumor embolization with injection of Onyx in a single session between 2013–2018. All cases displayed tumor arterial supply from ECA and ICA circulations on 2-D catheter angiograms. Procedural and surgical outcome data were analyzed. Results are given as mean±standard deviation (range). Among 9 patients with JNA, all were males and mean age was 14.1±6.3 years (range, 9–29 years). The mean tumor volume embolization was 84.4±12.4% (range, 60–100%) and in 89% patients ≥80% of tumor volume embolization was achieved. There were no embolization-related complications reported. During surgical resection of the tumor there was a low average surgical blood loss of 722±651.5 mL (range, 50–2,000 mL) and the mean procedure time was 282.6±85.4 mins (range, 151–403 mins). In this series, the BAE technique showed to be a safe and effective approach to achieve successful tumor embolization while avoiding embolic complications and effectively reducing the risk for blood loss during surgical resection.
Juvenile nasopharyngeal angiofibroma (JNA) is a rare, highly vascularized tumor that presents predominantly in males during the second decade of life. Typically located in the superior nasal cavity, the tumor often invades into the base of the skull and adjacent structures. Based on its histological features, JNA is commonly described as a neoplasm, but due to its hypervascularity and lack of a true capsule, surgeons often consider this tumor as more akin to a vascular malformation.1) Its complex network of arterial supply may arise from up to 10 potential branches of the external carotid artery (ECA) and internal carotid artery (ICA) circulation, including ECA-ICA anastomoses.2)16)19)
The ECA-ICA anastomoses directly involved in the vascular supply of this tumor may lead to inadvertent injection of embolic material into the ICA circulation by anterograde crossing from the ECA branches through the tumor feeders.3)5)7)11–13)18)20) Presence of these tumor feeders from ECA-ICA anastomoses has led operators to refrain from pursuing a more aggressive tumor embolization due to the risk of potential embolic strokes.21)
Here, we describe our experience in embolizing JNA tumors using a balloon-assisted embolization (BAE) technique in which a Hyperglide balloon in the C2-C4 ICA segments is simultaneously inflated during injection of Onyx into the tumor feeders. The goal of such technique is to avoid embolic complications that arise from shunting of the embolic material from the tumor feeders into the ICA circulation, thus providing a safe and effective approach for tumor embolization.
We retrospectively reviewed 9 consecutive cases of JNA patients that underwent pre-operative intra-arterial tumor embolization followed by surgical resection between 2013–2018. All tumors displayed tumor feeders from ECA-ICA anastomoses, were embolized using Onyx-34, and were performed by a single neurointerventionist. Data recorded included age, sex, ethnicity, clinical presentation, Radkowski stage, tumor size, tumor arterial feeders, tumor volume embolization, extent of resection, average surgical blood loss (ASBL), transfusion details, surgical procedure time, and embolization-related and post-operative complications. The assessment of the Radkowski stage has been described previously.17) Tumor volume embolization was evaluated on 2-D digital subtraction angiography images by performing angiographic runs of the ECA at the start and at end of the embolization procedure. An independent blinded reader measured the tumor volume before (Pre-V) and after (Post-V) the procedure using the formula V=(A×B×C)/3, which has been previously described.14) The tumor volume embolization was the calculated as [Pre-V] − [Post-V]/Pre-V. Need for surgical reintervention was determined by recurrence of tumor or symptoms. This study was approved by the Institutional Review Board.
Under general anesthesia, femoral access is gained on both sides using a 6-French (6F) sheath. After performing 2-D angiographic runs of the ECA and ICA bilaterally, a 6F Envoy DA guiding catheter is advanced and placed in the ICA ipsilateral to the tumor feeders. Heparinization is used to prevent thrombotic complications, maintaining an activated clotting time between 200–300 seconds. A 5×30 mm Hyperglide balloon (Medtronic, Minneapolis, MN, USA) with a microwire is then introduced through the guiding catheter and placed at the carotid siphon, extending through the ICA C2-C4 segments. A second 6F Envoy DA catheter (DePuy Synthes, Raynham, MA, USA) is then advanced and placed into the ipsilateral ECA. A dual lumen micro-balloon catheter, Scepter balloon (Terumo, Tokyo, Japan) loaded with Traxcess 014 microwire (Terumo, Tokyo, Japan), is then inserted into the tumor feeder of interest. 2-D angiographic runs are performed to confirm maximal distal positioning of the balloon microcatheter tip. The 5×30 mm Hyperglide in the ICA is inflated between 2–5 minutes to protect the ICA circulation during injection of the embolic material. The micro-balloon catheter injection lumen is then primed with Onyx-34 (Medtronic, Minneapolis, MN, USA), the Scepter balloon is inflated, and Onyx-34 is slowly infused using a blank roadmap visualization to achieve maximal distal penetration. The Scepter balloon is deflated and a 2-D angiography run is performed to evaluate the extent of tumor volume embolization. The Hyperglide balloon is then deflated to restore flow in the ICA circulation, followed by removal of the Scepter balloon in the ECA. This process is then repeated until tumor vascular contrast blush is no longer present or embolization of all tumor feeders from the ECA was achieved.
Following embolization of tumor feeders from the ECA, a 2-D angiographic run of the ICA is obtained to identify tumor feeders from the ICA. In such case, a Hyperglide balloon is inflated just distal to the arterial pedicle to insert a dual micro-balloon catheter into the tumor feeder of interest. Once the catheter is placed in the maximal distal segment the wire is removed, and the balloon is deflated and withdrawn slightly to ensure coverage of the feeder base in order to prevent liquid embolic material to reflux back to the ICA. The protective balloon is then inflated sealing the pedicle from the ICA to prevent reflux. Onyx-34 is slowly injected into the ICA feeder under complete balloon seal via blank roadmap technique allowing for safe and effective penetration of the embolic material. The microcatheter is removed, followed by balloon deflation. At the end of the procedure, injection of the ECA and ICA is performed to assess the percentage of tumor volume embolized. Please refer to Fig. 1 for an illustrative representation of this technique.
Among 9 patients included in this series, all were males and mean age was 14.1±6.3 years (range, 9–29 years). Epistaxis was the most common symptom at the initial presentation (89%) followed by nasal obstruction (56%), and a 44% of tumors were Radkowski stage 2b. All cases displayed tumor arterial supply from ECA and ICA circulations on 2-D catheter angiograms, with a total number of arterial tumor feeders embolized in a given session ranging between 2–6.
The mean tumor volume embolization was 84.4±12.4% (range, 60–100%), with subsequent gross total and subtotal resection achieved in 89% of the cases. A complete tumor volume embolization was achieved in Cases 7 and 8 and had a Radkowski stage 2a and 2b, respectively. Mean ASBL was 722 mL (range, 50–2,000 mL), and in the last 6 cases was 333 mL (range, 50–600 mL). Mean surgical procedure time was 282.6 mins (±85.4 mins). One case underwent a second embolization for tumor recurrence 14 months after the initial surgery (Case 5) and one case required a repeat embolization session to address residual tumor 4 years after the initial procedure (Case 6). One patient experienced late-onset, transient facial palsy as a surgical-related complication (Case 2).
JNA is a rare tumor usually presenting among young adolescent males. Although considered as a benign tumor, it has a locally aggressive behavior invading adjacent structures and eroding bone, thus carrying a significant morbidity during surgical resection.1) The tumor is composed by a complex matrix of blood vessels with a single endothelial lining surrounded by fibrous stroma, resulting in a highly fragile mass with a significant risk of massive bleeding with even minimal manipulation.
In JNA, tumor feeders tend to arise from the ECA by two of its branches: the internal maxillary artery and the ascending pharyngeal artery. However, upregulated expression of vascular growth factors induces tumor growth and consequentially blood supply from segments or branches of the ICA, from which ECA-ICA anastomoses often develop. Tumors feeders that exhibit shunting into the ICA circulation through these anastomoses are recommended to be embolized using the BAE technique to ensure the safety and efficacy of the procedure. Otherwise, inadvertent injection of these anastomoses may result in embolic complications with potential cranial nerve lesions and vision loss.5)7)9)11–13)15)18)20)
The complexity of the vascular supply of JNA coupled with its intraoperative-associated morbidity has led to the use of endovascular therapy to perform preoperative embolization, as first described in 1979.8) Preoperative tumor embolization using embolic agents (e.g., Onyx) has shown to decrease operative times and blood loss.10) At our institution, Onyx is the preferred embolic agent for tumor embolization, as it provides a deeper penetration to tumor capillaries, improved fluoroscopic visibility, and lower risk of catheter adherence and secondary vessel injury. However, one of most significant risk of any embolic material is inadvertent injection into the arterial system. Although the use of a percutaneous tumor puncture (i.e., direct tumor puncture)4) may allow for better visibility during injection of the embolic material, its benefit is outweighed by the potential migration of embolic material into ICA branches through ECA anastomoses.3)5)
This scenario represents a challenge to neurointerventionists, as the risk of embolizing an ICA branch through ECA-ICA anastomoses hinders a complete tumor embolization, leading to a significant risk of blood loss during surgical resection. Use of the BAE technique at our institution has allowed us to overcome these issues. Additionally, balloon protection of the ICA circulation while injecting embolic material through the ECA facilitates obliteration of tumor feeders that would have remained patent otherwise. In our series, there were no complications from inadvertent embolization of arterial vessels, embolic material entering the ICA circulation, or ophthalmic artery involvement.
We reported a high tumor volume embolization of 84% with over 90% of tumor volume embolization was achieved in four cases, contrary to other reports where successful tumor devascularization was only achieved with direct tumor puncture.3) Despite a prolonged procedure time when selectively embolizing tumor feeders as opposed to direct tumor puncture, the benefit from controlling the injection of the embolic material in our approach outweighs the potential risks of increasing the procedure time. In addition, direct tumor puncture has its own risks that cannot be disregarded. Of note, radiation dose and fluoroscopy time were within our institution’s standards. Furthermore, injection of Onyx with concurrent balloon protection of the ICA reduces procedure time when compared to the traditional trans-arterial approach, as fewer tumor feeders will need to be individually selected for embolization.
The use of simultaneous balloon occlusion across the ophthalmic and petrous segments of the ICA is crucial to safely embolize the tumor feeders, but is not without its own risks. There is a potential risk for vascular injury, such as perforation or iatrogenic dissection of the vessel which may lead to hemorrhagic or ischemic complications, respectively. A study analyzing risks for ischemic complications after a carotid sacrifice procedure with balloon occlusion revealed acute hemodynamic changes in blood pressure and heart rate when the balloon was inflated,6) as fluctuating hemodynamic parameters may pose a risk for hypoperfusion ischemia. Due to its potential risks, baseline blood pressure, the effects of the anesthetic agent on compensatory regulatory mechanisms, and occlusion time of ICA should all be considered during tumor embolization and simultaneous balloon occlusion (SBO). JNA patients, typically young males without pre-existing medical co-morbidities, are more likely to tolerate SBO through collateral flow through the anterior communicating artery or ipsilateral posterior communicating artery, if present. In our series, no patients in our cohort developed ischemia secondary to hypoperfusion from SBO.
In our series, use of the BAE technique resulted in low-risk blood loss during surgical resection as shown by the mean ASBL of 722 mL (range, 50–2,000 mL), lower than the intraoperative blood loss of 862 mL reported by Elhammady et al.9) in their case series of JNA tumors treated by transarterial embolization. Further, mean ASBL was reduced to 333 mL within one year, a reduction of 78%, and lower than reported in the literature. In severe cases, given the extent tumor invasion to bone structures, significant blood loss can be expected even if the tumor is completely embolized. The amount of ABSL in high Radkowski stage cases may be influenced by the involvement of intracranial structures, although experience of the resecting surgeon cannot be ignored. Certainly, embolization has proven helpful even in highly aggressive cases, as shown in Case 3 with a tumor size of 85 mm×69 mm×47 mm and a Radkowski stage 2c. Strategic selection of critical tumor feeders from the ICA circulation, such as the meningohypophyseal trunk (Case 4), may also serve in predicting ASBL.
Our case series continue to provide evidence on the safety and efficacy of the BAE technique in tumor embolization of JNA. Furthermore, the concept of balloon-assisted protection to embolize complex, highly vascularized tumors may be applied in other endovascular surgical situations, warranting further research.
Currently, most interventionists continue to embolize JNA tumors without considering the use of balloon protection of the ICA from the embolic material in the setting of ECA-ICA anastomoses, leading to surgical complications and incomplete embolization. In this study we provide more strength to the argument that the BAE technique is a safe and effective approach to perform successful embolization, eliminating the risk of embolic stroke and continuing to reduce blood loss during surgery.
REFERENCES
1. Beham A, BehamSchmid C, Regauer S, Auböck L, Stammberger H. Nasopharyngeal Angiofibroma: True Neoplasm or Vascular Malformation? Adv Anat Pathol. 2000; Jan. 7(1):36–46.

2. Berenstein A, Lasjaunias P, Kricheff II. Functional anatomy of the facial vasculature in pathologic conditions and its therapeutic application. AJNR Am J Neuroradiol. 1983; Mar–Apr. 4(2):149–53.
3. Borota L, Mahmoud E, Nyberg C, Ekberg T. Combined percutaneous and transarterial devascularisation of juvenile nasopharyngeal angiofibroma with protection of internal carotid artery: A modification of the technique. Interv Neuroradiol. 2015; Jun. 21(3):390–6.

4. Casasco A, Herbreteau D, Houdart E, George B, Tran Ba, Huy P, Deffresne D, et al. Devascularization of craniofacial tumors by percutaneous tumor puncture. AJNR Am J Neuroradiol. 1994; Aug. 15(7):1233–9.
5. Casasco A, Houdart E, Biondi A, Jhaveri HS, Herbreteau D, Aymard A, et al. Major complications of percutaneous embolization of skull-base tumors. AJNR Am J Neuroradiol. 1999; Jan. 20(1):179–81.
6. Chen P, Ortiz R, Page JH, Siddiqui AH, Veznedaroglu E, Rosenwasser RH. Spontaneous systolic blood pressure elevation during temporary balloon occlusion increases the risk of ischemic events after carotid artery occlusion. Neurosurgery. 2008; Aug. 63(2):256–64.

7. Countee RW, Vijayanathan T. External carotid artery in internal carotid artery occlusion. Angiographic, therapeutic, and prognostic considerations. Stroke. 1979; Jul. 10(4):450–60.

8. Coyas A, Tzagarakis M, Giorgis G, Katsiotis P. Transcatheter arterial embolization as a preoperative procedure in the management of juvenile angiofibroma. Rhinology. 1979; Dec. 17(4):265–70.
9. Elhammady MS, Johnson JN, Peterson EC, Aziz-Sultan MA. Preoperative embolization of juvenile nasopharyngeal angiofibromas: transarterial versus direct tumoral puncture. World Neurosurg. 2011; Sep–Oct. 76(3–4):328–34.

10. Gao M, Gemmete JJ, Chaudhary N, Pandey AS, Sullivan SE, McKean EL, et al. A comparison of particulate and onyx embolization in preoperative devascularization of juvenile nasopharyngeal angiofibromas. Neuroradiology. 2013; Sep. 55(9):1089–96.

11. Gay I, Elidan J, Gordon R. Oronasal fistula–a possible complication of pre-operation embolization in the management of juvenile nasopharyngeal angiofibroma. J Laryngol Otol. 1983; Jul. 97(7):651–6.

12. Geibprasert S, Pongpech S, Armstrong D, Krings T. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol. 2009; Sep. 30(8):1459–68.

13. Krishnamoorthy T, Gupta AK, Rajan JE, Thomas B. Stroke from delayed embolization of polymerized glue following percutaneous direct injection of a carotid body tumor. Korean J Radiol. 2007; May–Jun. 8(3):249–53.

14. Monga SP, Wadleigh R, Sharma A, Adib H, Strader D, Singh G, et al. Intratumoral therapy of cisplatin/epinephrine injectable gel for palliation in patients with obstructive esophageal cancer. Am J Clin Oncol. 2000; Aug. 23(4):386–92.

15. Naithani P, Khanduja S, Sinha S, Khanduja N, Naithani P. n-Butyl cyanoacrylate-induced multiple retinal arteriolar occlusions. Int Ophthalmol. 2013; Oct. 33(5):599–600.

16. Perrini P, Cardia A, Fraser K, Lanzino G. A microsurgical study of the anatomy and course of the ophthalmic artery and its possibly dangerous anastomoses. J Neurosurg. 2007; Jan. 106(1):142–50.

17. Radkowski D, McGill T, Healy GB, Ohlms L, Jones DT. Angiofibroma. Changes in staging and treatment. Arch Otolaryngol Head Neck Surg. 1996; Feb. 122(2):122–9.

18. Ramezani A, Haghighatkhah H, Moghadasi H, Taheri M, Parsafar H. A case of central retinal artery occlusion following embolization procedure for juvenile nasopharyngeal angiofibroma. Indian J Ophthalmol. 2010; Sep–Oct. 58(5):419–21.

19. Siddiqui AH, Chen PR. Intracranial collateral anastomoses: relevance to endovascular procedures. Neurosurg Clin N Am. 2009; Jul. 20(3):279–96.

20. Trivedi M, Desai RJ, Potdar NA, Shinde CA, Ukirde V, Bhuta M, et al. Vision loss due to central retinal artery occlusion following embolization in a case of a giant juvenile nasopharyngeal angiofibroma. J Craniofac Surg. 2015; Jul. 26(5):e451–3.

21. Valavanis A. Preoperative embolization of the head and neck: indications, patient selection, goals, and precautions. AJNR Am J Neuroradiol. 1986; Sep–Oct. 7(5):943–52.
Fig. 1
Illustration demonstrating example of catheter and balloon placement prior to (A) and after (B) infusion of Onyx in tumor feeders resulting in complete embolization of tumor vasculature. Black arrow: The 5×30 mm Hyperglide™ balloon in the ICA is inflated, thus protecting the C2-C4 segments of the ICA and their branches (single, double arrow heads). White arrow: Scepter™ balloon is inflated in ECA for selective Onyx-34 injection. Curved arrow: Duo™ microcatheter loaded with an 0.10 microwire to be inserted into arterial feeder of interest using the proximal end of balloon to assist in guiding wire into arterial trunk. ICA, internal carotid artery; ECA, external carotid artery.
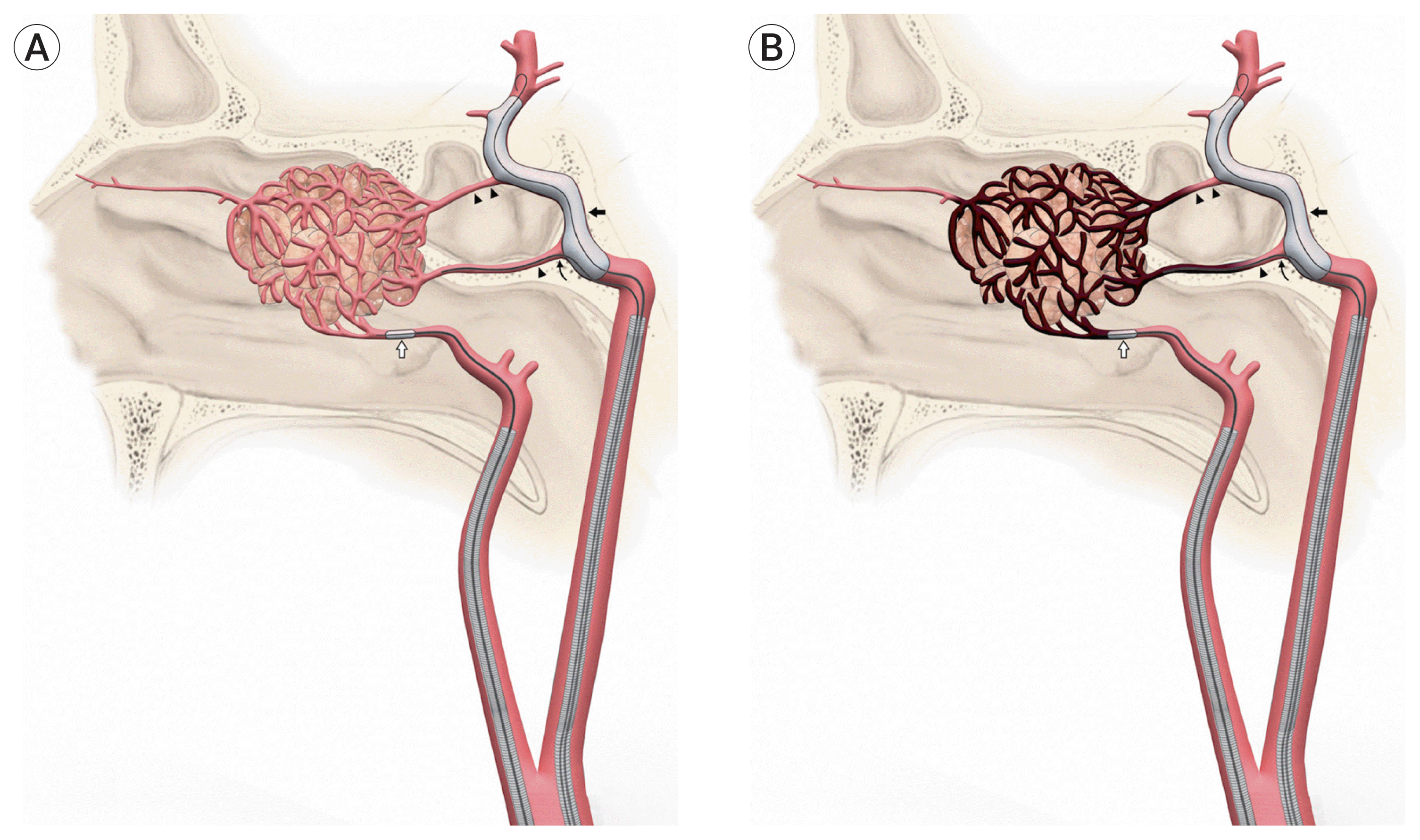
Fig. 2
Demonstrative case, Part 1: (A) Pre-embolization subtracted angiogram of left ICA and left ECA circulations (coronal and sagittal views) shows a large left posterior nasal cavity tumor measuring 43.5 mm×44.1 mm×55.7 mm with a blood supply from distal branches of the left sphenopalatine artery, left accessory meningeal artery, left middle meningeal artery, left inferior lateral trunk, and left vidian artery. (B) Embolization of ECA feeders (balloon inflated but not visualized). (C) Unsubtracted angiogram showing Onyx cast with evidence of deep penetration to peripheral feeders. (D) Subtracted angiogram of ICA after ECA embolization shows persistent vascularization supplied by vidian artery. (E) Roadmap showing microcatheter in distal vidian artery protected by balloon. (F) Embolization of Vidian artery feeders under balloon protection. (G) Post-embolization subtracted angiogram shows reduction of tumor blush by >90%. ICA, internal carotid artery; ECA, external carotid artery.
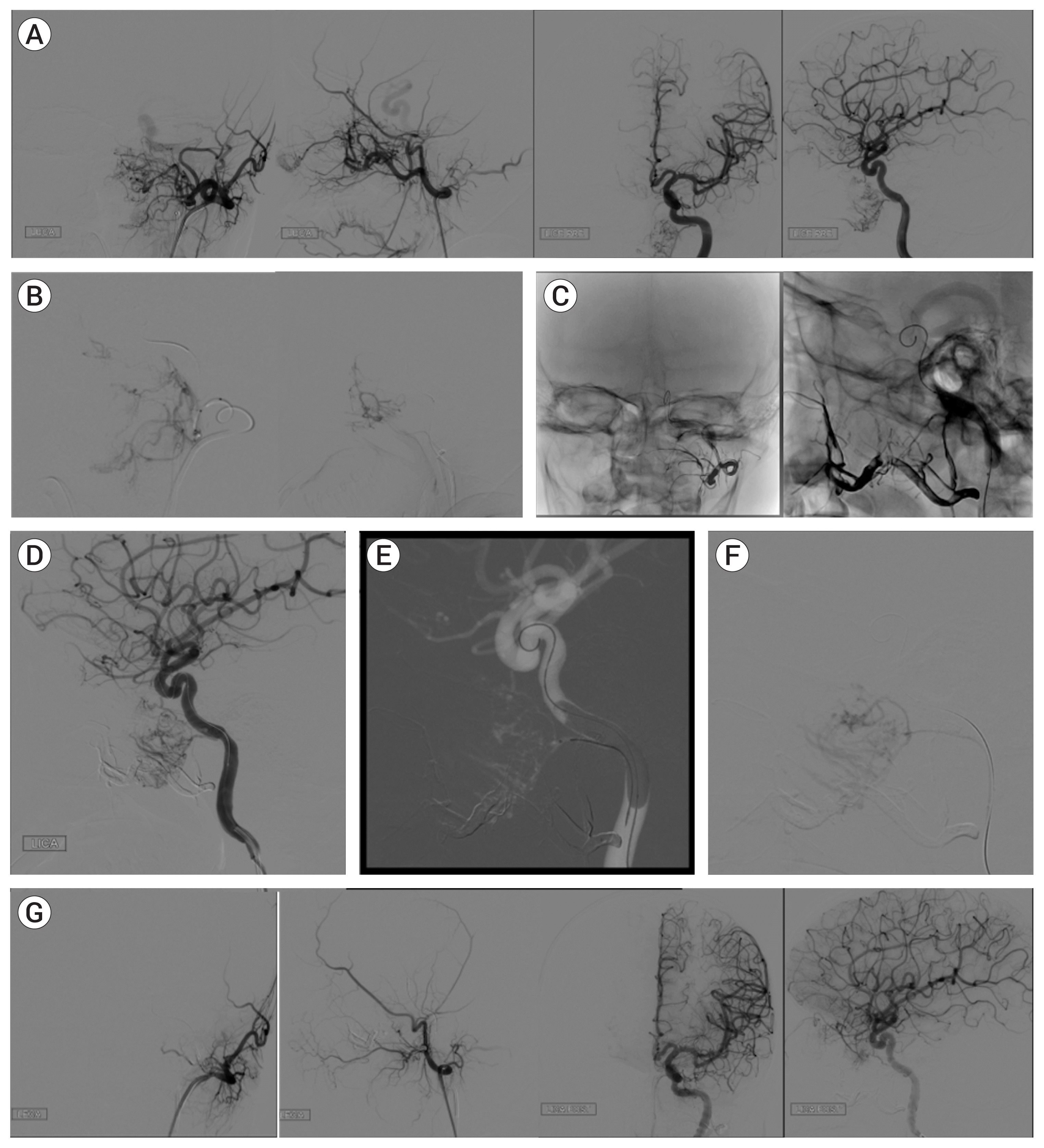
Fig. 3
Demonstrative case, Part 2: (A) Pre-embolization subtracted angiogram after ICA injection demonstrates recurrent tumor supplied by inferior lateral trunk. (B) Roadmap showing placement of microcatheter in distal inferior lateral trunk with ICA balloon inflated for protection. (C) Post-embolization subtracted ICA injection shows blush reduction of 90% and abrupt cut-off of inferior lateral trunk flow to tumor. ICA, internal carotid artery.
Table 1
Clinical and procedural characteristics of patients treated in the cohort




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download