This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
Mechanical thrombectomy (MT) is now an established treatment for acute ischemic stroke (AIS) with large vessel occlusion (LVO) within 6 hours. Since 2018, MT is also recommended from 6–24 hours after selecting with additional multimodal imaging including perfusion imaging. We sought to investigate patients with significant discrepancy in core infarct between computed tomography (CT) and CT perfusion (CTP).
Methods
In this retrospective study, patients with AIS who were evaluated for MT using the RAPID software (IschemaView, Redwood City, CA, USA) from February 2018 to March 2019 were included. Cases with discrepancy between infarct volume on non-contrast CT and core volume (cerebral blood flow <30%) as analyzed by RAPID on CTP were analyzed.
Results
In the study period, 635 patients were evaluated for acute stroke symptoms. Non-contrast head CT was performed in 635 patients, and CTP with RAPID software post processing was performed in 134 patients. Among the 134 patients, 8 (5.9%) patients had gross discrepancy in core infarct between CT and CTP, with underestimation of infarct by CTP. Evaluation of these cases shows that the likely reason for this discrepancy is recanalization of a LVO, which then leads to erroneously normal or gross underestimate of the core infarct volume determined from CTP post processing analysis.
Conclusions
Recanalization of a LVO can lead to erroneously normal or gross underestimation of the core infarct as determined by post processing software analysis of CTP data. The whole composite of hyperacute CT imaging should be examined while making decisions. This caveat of perfusion imaging interpretation has not been reported previously.
Go to :

Keywords: Stroke, Thrombectomy, CT perfusion, Core, Discrepancy
INTRODUCTION
Mechanical thrombectomy (MT) is now an established treatment for acute ischemic stroke (AIS) from large vessel occlusion (LVO) of the first segment of the middle cerebral artery (MCA) or internal carotid artery (ICA) within 6 hours.
7) After the publication of the DAWN (DWI or computed tomographic perfusion (CTP) Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo)
5) and DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke)
1) trials in 2018, MT is now also recommended in selected patients with LVO in the 6–24 hours time period.
7) These trials used additional multimodal imaging to select patients for MT, including perfusion imaging to detect clinical-imaging mismatch (DAWN trial) or perfusion-core mismatch (DEFUSE 3 trial). Most recently, CTP has also been used to guide intravenous thrombolysis up to 9 hours by selecting patients with salvageable brain tissue.
4) All these trials used an automated image post processing system, RAPID Imaging software (IschemaView, Redwood City, CA, USA) to calculate the volume of the ischemic core and penumbra. Since the adoption of above guidelines, our center has added CTP in addition to non-contrast head computed tomography (CT) and CT angiography (CTA) of the head and neck to select patients for MT. We sought to investigate patients with significant discrepancy in core infarct between CT and CTP.
Go to :

MATERIALS AND METHODS
In this retrospective study, we analyzed data from patients with AIS who were evaluated for MT using the RAPID software from February 2018 to March 2019 at our comprehensive stroke center. Institutional Review Board approval was obtained prior to performance of the study. All patients with stroke symptom onset within 24 hours were evaluated for possible MT with non-contrast head CT, and CTA head and neck. Standard guidelines for intravenous thrombolysis were followed. For patients with M1 MCA and/or ICA occlusion within 6 hours, selection for MT was mostly made based on clinical severity and Alberta Stroke Program Early CT (ASPECT) score. CTP was not necessary but was performed in selected cases at the discretion of the treating team, for wake up strokes, or when significant early ischemic changes/hypodensity was seen on CT. For patients with M1 MCA and/or ICA occlusion in the 6–24 hours time window, CTP was always performed. For other vessel occlusions including M2 MCA, vertebra-basilar, or P1 posterior cerebral artery (PCA), decision for MT was made on a case by case basis. Not all patients for MT were selected according to strict criteria laid out in clinical trials; and thus, it reflects the biases and preferences of real-world practice.
CTP image acquisition, post processing and analysis
All CT scans are obtained at our institution using multi–detector CT scanners including dual energy 128-slice and 64-slice Siemens scanners. After unenhanced CT of the whole brain and CTA head and neck, CT perfusion was performed soon after when deemed necessary. 40 mL nonionic contrast agent (Visipaque 320) is injected at the rate of 4 mL/sec followed by 20 mL normal saline at the rate of 4 mL/sec. At 6 seconds after the initiation of injection, a cine (continuous) scan is done with the following technique: 80 kVp, 150 mAs with scan time of 1.5 seconds per rotation. RAPID Imaging software was used to analyze the CTP data. Ischemic core is diagnosed if the relative cerebral blood flow (CBF) is <30% of that in normal brain tissue.
2) Hypoperfusion is distinguished from minimally hypoperfused tissue if the Tmax delay is >6 seconds.
3)6)8) Cases of severe motion artifacts or poor cardiac output generating erroneous CTP data were excluded.
Cases with discrepancy between infarct volume on non-contrast CT and core volume (CBF <30%) on CTP were analyzed. Discrepancy was defined as lack of core detection on CTP of >2 subcortical regions and/or >1 cortical region on ASPECT scoring of the non-contrast CT. In other words, if hypodensity in 2 or more subcortical regions, or 1 or more cortical region on non-contrast CT ASPECTS scoring was not detected by CTP CBF <30% summary maps, this was marked as a discrepancy. In case of concurrent posterior circulation involvement, only hypodensity in the occipital cortex was included for analysis.
Go to :

RESULTS
In the study period, 635 patients were evaluated for acute stroke symptoms. Non-contrast head CT was performed in 635 patients, CTA head and neck in 631 patients, and CTP with RAPID software post processing was performed in 134 patients. Among the 134 patients, 8 (5.9%) patients had gross discrepancy in core infarct between CT and CTP. In all these cases, there was gross underestimation of core infarct volume as determined by post processing analysis of CTP data.
Table 1 gives details on demographic, stroke timelines, stroke severity, treatment and imaging characteristics. The cohort included 4 (50%) females; with age ranging from 18–60 (median 38.5 years). Three patients were wake up strokes. Two patients presented <6 hours, 5 in the 6–24 hours time window, and 1 patient >24 hours from symptom onset. Six patients had isolated involvement of the MCA distribution. One patient had isolated involvement of the PCA distribution (Patient 5), and one patient with concurrent involvement of the MCA and PCA (Patient 8). There was no LVO in 5/8 patients. In the remaining 3 patients, there was occlusion of the distal P1 PCA, anterior division M2 MCA, and severe stenosis of the M1 and P1 without occlusion (Patient 6, 7, 8 respectively). Intravenous thrombolysis was administered in 3/8 patients. None of these patients underwent MT. With regards to discrepancy between CT and CTP, Patients 1–4 and 6 had obvious hypodensity in the MCA distribution, with ASPECTS ranging from 2–8, but no core (0 mL) detected by CTP post processing. Patient 5 had no MCA involvement (ASPECT 10) but obvious large occipital hypodensity which was not detected by CTP (0 mL). Patient 7 had ASPECT 5 and CTP detected 17 mL of core infarct in the distribution of the occluded anterior M2 distribution. However, the basal ganglia and posterior M2 division distribution hypodensity was not detected by CTP. Patient 8 had infarct in the MCA (ASPECT 7) and PCA distribution but core infract of 17 mL was only detected in the PCA distribution by the CTP. Examples of the discrepancy in core infarct as assessed by CT and CTP are shown in
Fig. 1 (Patient 3),
Fig. 2 (Patient 5) and
Fig. 3 (Patient 6).
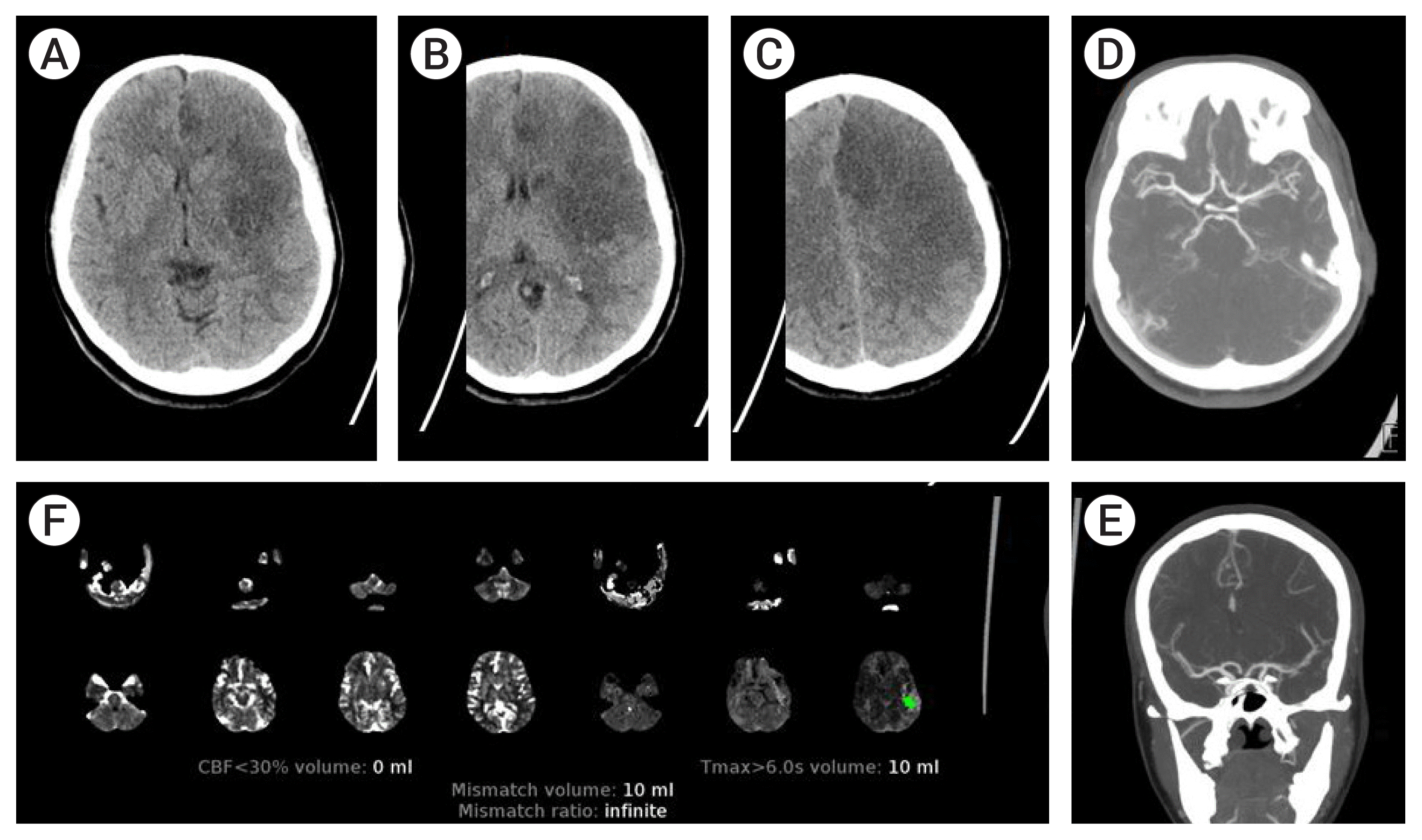 | Fig. 1Demonstration of core infarct discrepancy in patient 3. Axial non-contrast computed tomography (CT) head images (A, B, C) show large hypodense area in left basal ganglia, insular cortex and the frontal lobe. CT angiogram axial (D) and coronal (E) maximum intensity projection images show mild irregularity at the left middle cerebral artery (MCA) bifurcation, but patent left MCA vessels. CT perfusion summary map (F) shows 0 mL of core infarct in the left anterior hemisphere as calculated by cerebral blood flow (CBF) <30%. 
|
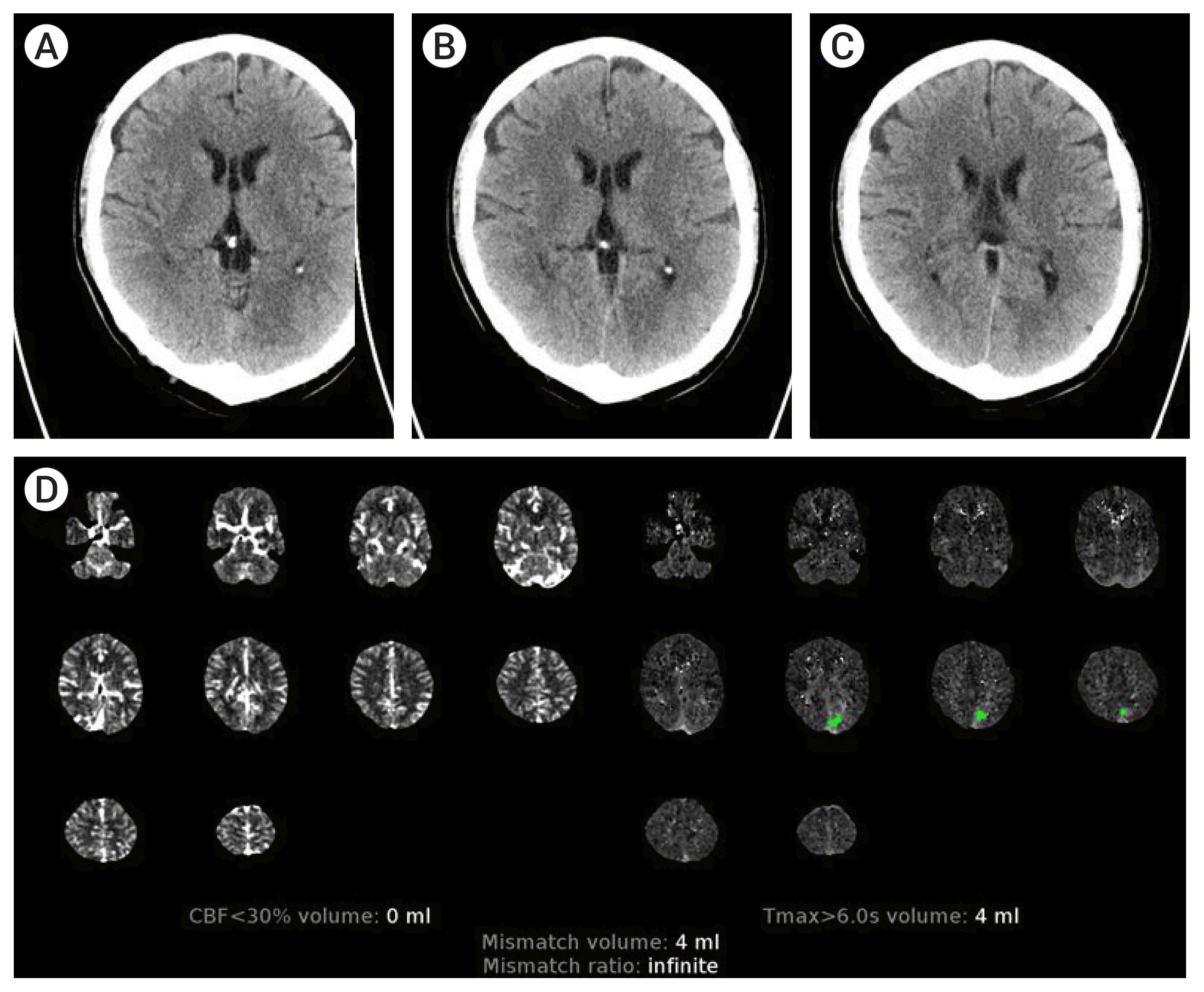 | Fig. 2Demonstration of core infarct discrepancy in patient 5. Axial non-contrast computed tomography (CT) head images (A, B, C) show hypodensity in the left occipital lobe in the distribution of the left posterior cerebral artery. CT angiogram in this case showed patent left posterior cerebral artery (PCA) (not shown). CT perfusion summary map (D) shows 0 mL of core infarct in the left PCA distribution as calculated by cerebral blood flow (CBF) <30%. 
|
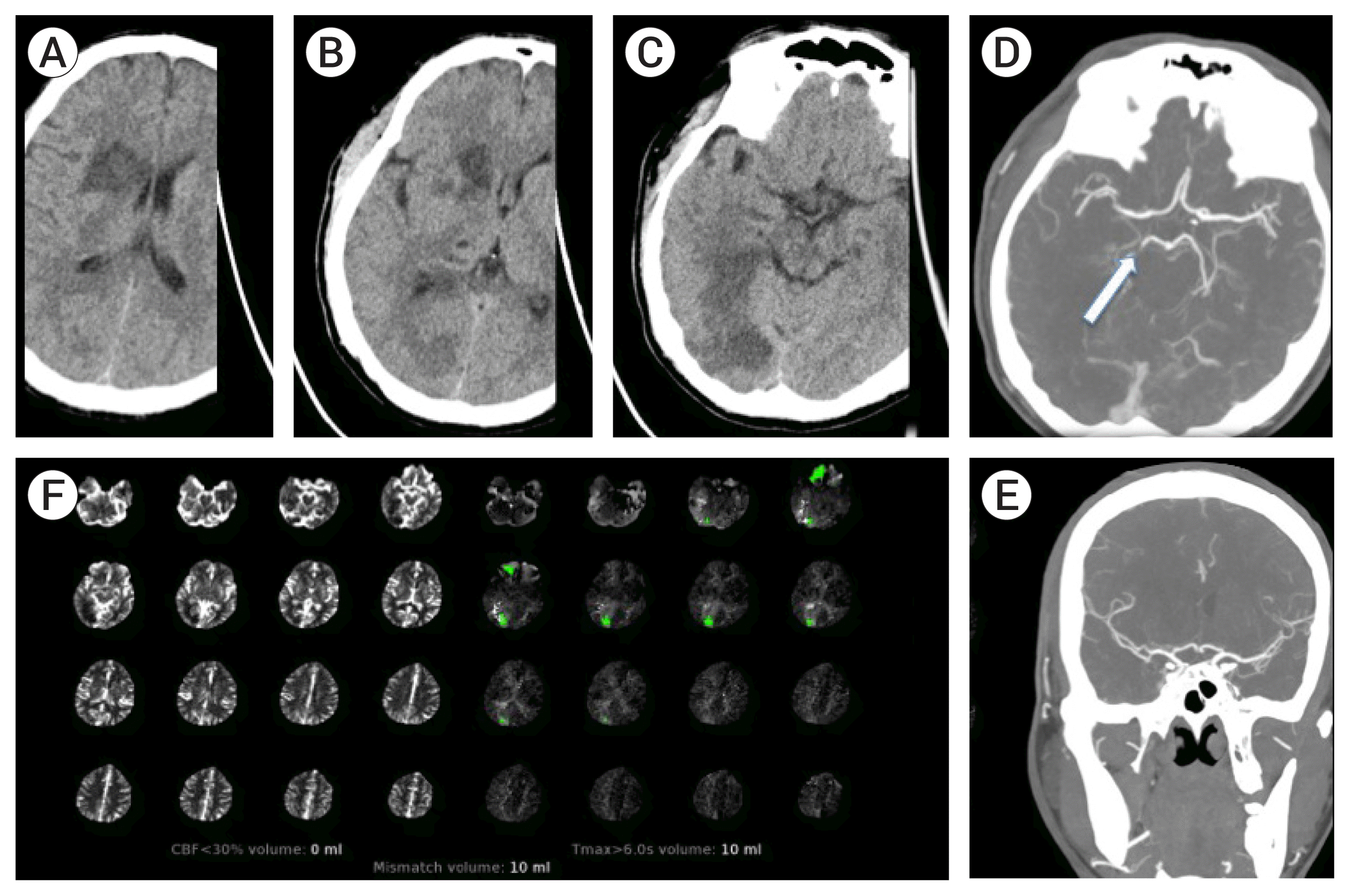 | Fig. 3Demonstration of core infarct discrepancy in patient 6. Axial non-contrast computed tomography (CT) head images (A, B, C) show clear hypodensity in the right periventricular area, basal ganglia and occipital lobe. CT angiogram axial (D) and coronal (E) maximum intensity projection images show patent right middle cerebral artery but a right P1 segment posterior cerebral artery occlusion (white arrow- D). CT perfusion summary map (F) shows 0 mL of core infarct as calculated by cerebral blood flow (CBF) <30%. 
|
Table 1
Patients with discrepancy in core infarct volume between CT and CTP detailing clinical characteristics, stroke time lines, and acute imaging findings on non-contrast CT, CTP and CTA
|
No. |
Age/Sex |
Wake up |
Time from last known well hh:mm |
Initial/Discharge NIHSS |
IV tPA |
Infarcted region |
LVO |
CT ASPECT score |
CBF <30% volume (mL) |
|
1 |
39/F |
Yes |
07:01 |
10/2 |
No |
RMCA |
No |
5 |
0 |
|
2 |
29/M |
No |
01:26 |
13/3 |
Yes |
RMCA |
No |
8 |
0 |
|
3 |
18/F |
No |
16:25 |
25/15 |
No |
LMCA |
No |
2 |
0 |
|
4 |
69/F |
Yes |
03:17 |
14/26 |
Yes |
LMCA*
|
No |
7 |
0 |
|
5 |
49/M |
No |
07:01 |
2/1 |
No |
LPCA |
No |
10 |
0 |
|
6 |
47/M |
Yes |
27:42 |
3/2 |
No |
RMCA and RPCA |
RP1 |
7 |
0 |
|
7 |
38/F |
No |
8:37 |
14/2 |
No |
LMCA |
LM2 ant division |
5 |
17 |
|
8 |
33/M |
No |
8:20 |
13/24 |
Yes |
RMCA+RPCA*
|
No LVO, Stenosis of RM1 and RP1 |
7 |
17 (RPCA) |

With regards to clinical follow-up, discharge NIHSS is detailed in the table adjacent to initial NIHSS. All patients stayed clinically stable or improved at discharge except Patient 4 and 8. Patient 4 suffered hemorrhagic transformation and additional ischemic infarcts secondary to atrial fibrillation. Patient 8 subsequently suffered a large left MCA ischemic infarct secondary to hypoperfusion from the severe M1 stenosis. As for imaging follow up, all patients received a repeat CT head or magnetic resonance imaging (MRI) of the brain within 48 hours, and at later time points as clinically indicated. There was no extension of infarct compared to initial imaging in all patients with the exception of Patient 4,8 (marked with asterisk in table). The reasons for increased infarct volume in Patients 4 and 8 are the same as that described in clinical follow up.
Go to :

DISCUSSION
The aim of this study was to assess if there is significant discrepancy in the core infarct as assessed by CT and CTP in AIS patients who are being evaluated for MT, and to determine the factors for the discrepancy. Our study demonstrated that approximately 6% of patients had significant discrepancy between the two modalities with underestimation of the core infarct by CTP summary maps. A careful evaluation of these cases shows that the likely reason for this discrepancy is recanalization of a LVO which then leads to erroneously normal or gross underestimate of the core infarct volume determined from post processing analysis. The automated CT brain perfusion analysis software detects infarct by measuring cerebral blood flow. If there is resolution of the occlusion after the development of infarct, this automated software cannot detect ischemic changes as the blood flow has been restored and the CBF is normal. To the best of our knowledge, this caveat of perfusion imaging interpretation has not been reported previously.
Analysis of patients 1–4 and 6 shows moderate to large infarct burdens on non-contrasted head CT that was likely caused from an M1 MCA occlusion. However, no LVO was detected, suggesting interval recanalization either spontaneously or secondary to intravenous thrombolysis. Consequently, the CTP completely failed to pick up any core, with CBF <30% of 0 mL in all these cases. Similarly, Patient 5 had a PCA infarct without a PCA occlusion likely from recanalization which led to an erroneously normal CBF map and consequently 0 mL of core infarct. In Patient 7, the CT shows infarction in the basal ganglia and territory supplied by both divisions of the M2 MCA. However, CTP only demonstrated core infarct in the distribution of the occluded anterior M2 division. It is likely that both the M1 MCA and posterior M2 division had recanalized by the time imaging occurred. Finally, Patient 8 had no LVO but did harbor severe stenosis of both the M1 and P1 with hypodensities in both vascular distributions on CT. However, CTP was unable to detect the MCA infarct (possibly secondary to MCA recanalization or adequate flow through the stenosis) and only revealed the PCA territory infarct.
These findings have practical implications. Estimation of the size of infarct is crucial in selecting patients for MT. Therefore, CTP images should always be interpreted alongside non-enhanced CT images. It is often felt that CTP imaging along with post processing analysis maps are easier to interpret compared to subtle findings seen on plain CT images. However, the whole composite of data should be evaluated together in order to avoid misinterpretation, such as in the case of interval recanalization of occluded vessels. This approach would better evaluate patients who may not benefit from MT, and conversely avoid eliminating patients who may benefit from endovascular treatments.
It could be argued that selection for MT will not be affected since it necessitates requirement of a LVO in the first place (i.e., MT is not a consideration if there is no LVO in the first place). However, the composite of CT, CTA and CTP is still valuable in selection of MT in more distal vessel occlusion much as distal M2 or M3 segments. This if often seen when there is an initial proximal vessel occlusion with subsequent recanalization and residual distal emboli. CTP data in these cases as described in out manuscript may be incorrect.
The shortcoming of CTP described here could certainly affect intravenous thrombolysis treatment utilizing perfusion imaging that will likely become standard of care in the future. The EXTEND trial
4) was the first positive trial utilizing intravenous thrombolysis in the extended time window in stroke patients with hypoperfused but salvageable regions of brain detected on automated perfusion imaging. The trial randomly assigned patients to receive intravenous alteplase or placebo between 4.5 and 9.0 hours after the onset of stroke or on awakening with stroke. The perfusion imaging inclusion criteria included and an ischemic-core volume of less than 70 mL, an absolute difference in volume greater than 10 mL, and perfusion lesion–ischemic core mismatch ratio greater than 1.2 between the volume of hypoperfusion and the volume of the ischemic core. With the caveat of perfusion imaging detailed in our report, it is easily possible for all above parameters to be skewed or incorrect, thereby influencing the thrombolysis decision if going by CTP parameters alone.
Our findings may have a bearing on the sequence of imaging in AIS. Some centers perform CTP imaging immediately after the plain CT, and lastly CTA head and neck based on CTP findings. If plain CT findings for ischemia are subtle or not extensive, a normal CTP for core assessment does not rule out infarction (possible recanalized vessel occlusion). In the same token, large burden of infarction on CT head with normal or low core by CTP analysis should alert the physician regarding interval LVO recanalization. A CTA should be part of hyperacute stroke imaging in either of the above scenarios.
This study has multiple limitations foremost of which include its retrospective design and small numbers. A CTP was performed in multiple cases despite lack of obvious M1 MCA or ICA occlusion. This was done because the hypodensity seen on plain CT prompted questions regarding occlusions of smaller distal vessels that were not apparent on CTA. Perfusion CT was then done to assess salvageable penumbra that may be amenable to MT or to see if multiple smaller distal vessels were compromised. Discrepancy between CT and CTP core assessment is also clearly influenced by collateral supply to distribution of the occluded vessel. For instance, Patient 6 had no infarct demonstrable by CTP in the PCA territory despite an acute P1 occlusion. This is likely secondary to exceptional collateral supply. Our study only selected patients with gross discrepancies between the two modalities. Smaller differences secondary to varying grades of collateral supply may have been missed.
Go to :

CONCLUSIONS
CTP imaging is an extremely useful modality in the hyperacute imaging of stroke, especially for selection of patients for MT. Our study has demonstrated that recanalization of a LVO can lead to erroneously normal or gross underestimation of the core infarct as determined by post processing software analysis of CTP data. The whole composite of hyperacute CT imaging should be examined while making decisions.
Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download