Abstract
Resection of biliary tract malignancies may require resection of the hepatic vasculature. While immediate revascularization of the liver is necessary, reconstruction is difficult when the original vessels are unavailable. We document a case in which a segment of the common hepatic artery was excised during tumor resection and the remaining proximal vessel displayed intima dissection. A greater saphenous vein was placed as a bridge between the remaining left hepatic artery and gastroduodenal artery for successful revascularization.
Go to : 
In hilar biliary tract malignancies, achieving negative surgical margins is greatly challenging due to the proximity of the liver and hepatic artery (HA) vasculature. Sufficient resection of the tumor may compromise arterial flow to the liver, and necessitate fabrication of the HA. Arterial reconstruction is also complicated by the factor of individual anatomical variations [1]. There have been studies reporting successful HA reconstruction using intra- or extra-abdominal vascular grafts during liver transplantation [2-5]. However, when there is an extensive vascular defect that is difficult to overcome with intra-abdominal methods, extra-abdominal grafts must be utilized. Here we report a case of left HA revascularization using an interpositional greater saphenous vein (GSV) graft bridge from the gastroduodenal artery after resection of intrahepatic cholangiocarcinoma.
Go to : 
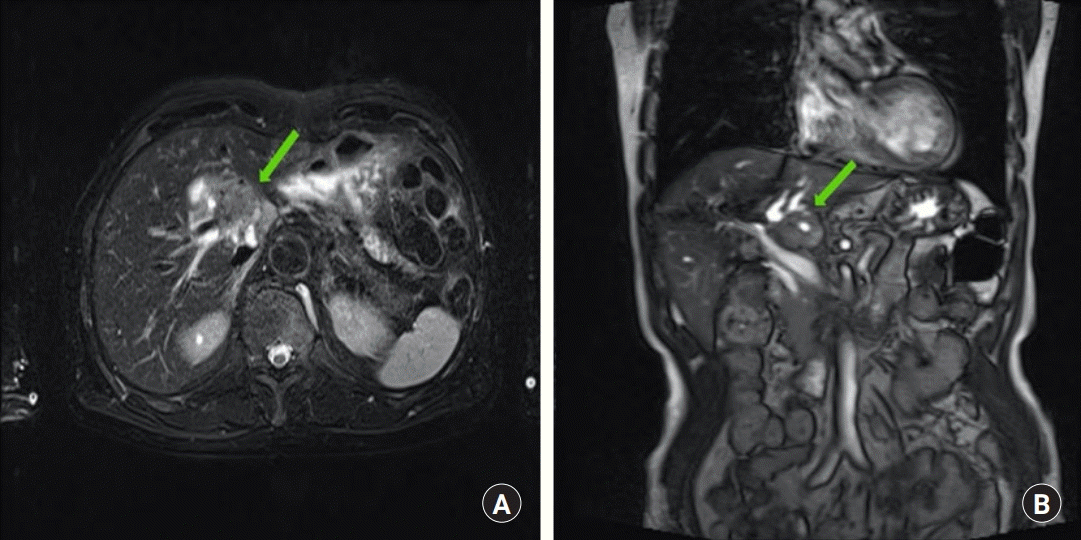
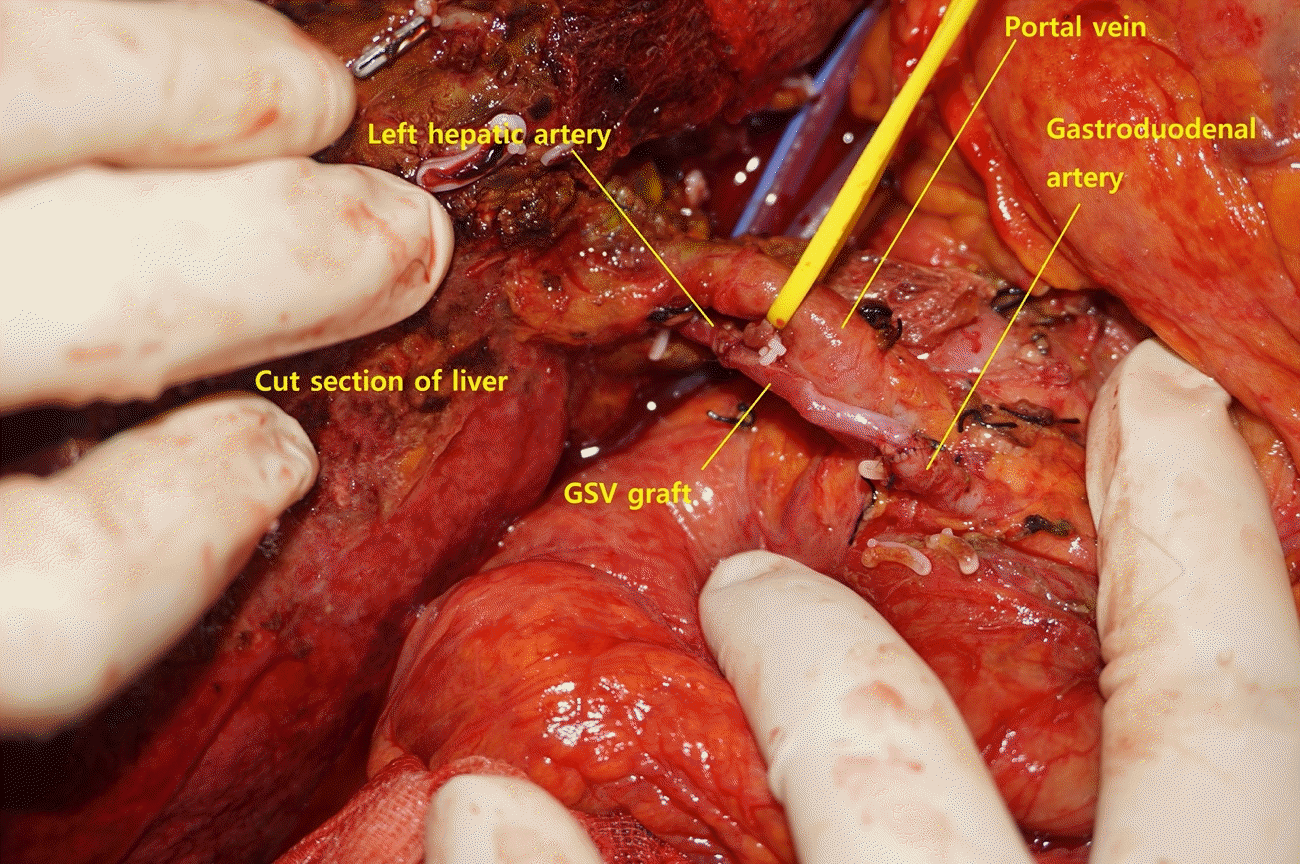
An 80-year-old male presenting with abdominal discomfort was diagnosed with cholangiocarcinoma. Magnetic resonance imaging revealed a 2.5-cm sized mass with delayed enhancement in the caudate lobe, with hilar involvement abutting the portal vein (Fig. 1). During open liver hemihepatectomy and cholecystectomy a 3-cm sized mass involving the caudate lobe, hepatic bifurcation, and perihilar lymph node enlargement was exposed. In the process of en bloc tumor resection around 2 cm of the left HA was excised, and the resulting arterial defect required reconstruction. Because the proximal stump of the proximal common HA revealed intimal dissection, attempts at in situ anastomosis between both margins of the defect were unsuccessful. Our team evaluated the gastroduodenal artery for a turnover arterial graft, however, much of the gastroduodenal artery also revealed intimal dissection, and after trimming to a healthy margin, the proximal stump was too short to be connected to the hepatic vasculature. A bridging vein graft from the GSV was planned. A portion of the left GSV 10 cm in length was harvested from the lower leg with loupes, and interposed to prevent obstruction at valves. Under microscope vision, after trimming to 3 cm, an end-to-end anastomosis was done between the left HA and the GSV graft using 8-0 ETHILON sutures first (Ethicon, Somerville, NJ, USA). Additional trimming to a length of 2.7 cm was done, and the proximal end of the GSV graft was anastomosed in the same manner to the gastroduodenal artery (Fig. 2). With removal of the vessel clamps, fresh bleeding increased at the cut surface of the hepatectomy. Empty-and-refill test and ultrasound Doppler were done and showed successful anastomosis.
Informed consent was obtained.
Go to : 
Radical surgical resection until a negative margin is secured provides a higher chance for cure and event free survival in advanced biliary cancer. However, the vascular anatomy in the hilar area is very complex, consisting of major vessels that directly supply vital organs, including the HA. Therefore vascular invasion of the biliary tumor is one of the main limitation factors that result in irresectability. Sufficient resection of the tumor along with the HA may compromise hepatic perfusion, and require HA reconstruction. Although Hu et al. [2] reported that HA resection during surgical excision of hilar cholangiocarcinoma may not require HA reconstruction in highly selected cases, most other studies have noted that failure to reconstruct the HA may lead to serious complications including hepatonecrosis or hepatic abscesses [2,6]. Thus, the surgeon must be equipped with the ability to fabricate the deficient HA.
When the arterial defect is smaller than 1 cm, the remaining HA can be dissected and advanced from the surrounding tissue. Anatomical variations of the HA vasculature should be contemplated. While the majority (80%) of cases are reported to have normal (Michels type I) HA anatomy, the remaining 20% may express one of numerous variations (Michels type II, replaced left HA from the left gastric artery; Michels type III, replaced right HA from the superior mesenteric artery; Michels type IV, replaced right HA and left HA; Michels type V, accessory left HA; Michels type VI, accessory right HA; Michels type VII, accessory right HA and left HA; Michels type VIII, replaced right HA or left HA with other HA being accessory one; Michels type IX, hepatic trunk as a branch of the superior mesenteric artery; and Michels type X, the common HA from the left gastric artery). A replaced right HA or left HA may be found, while accessory right and left HA occur in 0.8%–8% of patients [1,7,8]. Larger defects may be reconstructed using intra- or extra-abdominal vessels. Intra-abdominal vascular reconstruction requires knowledge on the various proximal arteries that may be utilized within the area. Extra-anatomic transposition that origin from the celiac trunk such as the right gastroepiploic artery, the gastroduodenal artery, the left gastric artery, or the splenic artery may be used for reconstruction. Defects larger than 2 cm will warrant a vessel graft. Intra-abdominal arterial grafts from the gastroduodenal artery, inferior mesenteric artery, iliac artery, splenic artery, and branches of the celiac trunk may be harvested [3-5].
When manipulation of the abdominal vasculature is unsafe and unwanted, the surgeon may opt to use an extra-abdominal or ectopic autologous graft. Reports on the use of the radial artery and the brachiocephalic artery for intraabdominal vascular reconstruction have been reported [9,10]. However, harvesting a major artery is inevitably accompanied by donor morbidity, and may be limited in availability. The GSV graft is a reliable autologous vessel graft donor, with less anatomical variation and minimal donor morbidity. It is a readily available option that is swiftly and easily harvested with a diameter that is similar to the hepatic vasculature. Bhatti et al. [5] have compared the outcomes of GSV grafts to arterial reconstruction using native HAs, and found that patency was excellent, though GSV grafts did have a higher rate of complications.
Although not optimal, the GSV graft is a reliable option that may be utilized in cases where the native hepatic vasculature has been sacrificed, or when the surrounding arteries are difficult to use. Much care should be taken to preserve the normal anatomy, however this should not be a restricting factor for tumor resection. To ensure radical resection of hilar malignancies of the biliary tract, the surgeon should be equipped with a solution for revascularization of the HA.
Go to : 
REFERENCES
1. Fonseca-Neto OC, Lima HC, Rabelo P, Melo PS, Amorim AG, Lacerda CM. Anatomic variations of hepatic artery: a study in 479 liver transplantations. Arq Bras Cir Dig. 2017; 30:35–7.

2. Hu HJ, Jin YW, Zhou RX, et al. Hepatic artery resection for bismuth type III and IV hilar cholangiocarcinoma: is reconstruction always required? J Gastrointest Surg. 2018; 22:1204–12.

3. Ozer A, Aktas H, Eren N, Karakayali H, Emiroglu R. Hepatic arterial reconstruction using right gastroepiploic artery in living donor liver transplantation. Transplant Proc. 2018; 50:3559–61.

4. Del Gaudio M, Grazi GL, Ercolani G, et al. Outcome of hepatic artery reconstruction in liver transplantation with an iliac arterial interposition graft. Clin Transplant. 2005; 19:399–405.

5. Bhatti AB, Dar FS, Qureshi AI, Haider S, Khan NA. Saphenous vein conduits for hepatic arterial reconstruction in living donor liver transplantation. Langenbecks Arch Surg. 2019; 404:293–300.

6. Wang WL, Tang XF, Yao MY, et al. Safety and efficiency of left hemihepatectomy combined with hepatic artery resection for hilar cholangiocarcinoma with artery infiltration: report of 2 cases. Can J Surg. 2008; 51:305–7.
7. Noussios G, Dimitriou I, Chatzis I, Katsourakis A. The main anatomic variations of the hepatic artery and their importance in surgical practice: review of the literature. J Clin Med. Res. 2017; 9:248–52.
8. Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966; 112:337–47.

9. Lin TS, Yang JC, Chen CL. Hepatic artery reconstruction using radial artery interposition graft in living donor liver transplantation. Transpl Int. 2013; 26:e28–30.

10. Javerliat I, Pichon A, Glorion M, Coscas R, Goeau-Brissonniere O, Coggia M. A new technique for intra-abdominal arteries revascularization via extra-anatomic bypass from the brachiocephalic artery with a videoscopic retrosternal tunnel. J Vasc Surg. 2015; 62:256–8.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download