Abstract
Background
South Asians generally have an unfavourable metabolic phenotype compared with white Caucasians, including central obesity and insulin resistance. The Wnt protein family interacts with insulin signaling, and impaired Wnt signaling is associated with adiposity and type 2 diabetes mellitus. We aimed to investigate Wnt signaling in relation to insulin signaling in South Asians compared with white Caucasians.
Methods
Ten Dutch South Asian men with prediabetes and overweight or obesity and 10 matched Dutch white Caucasians were included. Blood samples were assayed for the Wnt inhibitor sclerostin. Subcutaneous white adipose tissue (WAT) and skeletal muscle biopsies were assayed for Wnt and insulin signaling gene expression with quantitative reverse transcription polymerase chain reaction (Clinicaltrials.gov
NCT02291458).
Results
Plasma sclerostin was markedly higher in South Asians compared with white Caucasians (+65%, P<0.01). Additionally, expression of multiple Wnt signaling genes and key insulin signaling genes were lower in WAT in South Asians compared with white Caucasians. Moreover, in WAT in both ethnicities, Wnt signaling gene expression strongly positively correlated with insulin signaling gene expression. In skeletal muscle, WNT10B expression in South Asians was lower, but expression of other Wnt signaling and insulin signaling genes was comparable between ethnicities. Wnt and insulin signaling gene expression also positively correlated in skeletal muscle, albeit less pronounced.
The incidence of type 2 diabetes mellitus is increasing worldwide and its morbidity and mortality rates are accompanied by a high socioeconomical burden [1]. In the South Asian population, originally deriving from the Indian subcontinent and comprising approximately one fifth of the world population, the prevalence of type 2 diabetes mellitus is particularly high [23]. This may at least partly be explained by a disadvantageous metabolic phenotype, including central obesity and insulin resistance [456]. The underlying cause of this metabolic phenotype is not yet fully elucidated, but may be related to a difference in energy metabolism favouring energy storage.
The Wnt family is well-known for its function in embryonic development and oncogenesis. The canonical route of this signal transduction pathway comprises endogenous Wnt ligands that bind to Wnt coreceptors named Frizzleds (FZD) and low-density lipoprotein receptor-related proteins 5 (LRP5) and 6 (LRP6). Upon binding of Wnt ligands to these receptors, β-catenin is rescued from being degraded, facilitating its nuclear translocation and ultimately inducing transcription of Wnt target genes [78].
Evidence has emerged that Wnt signaling also plays a role in metabolism, since an impairing mutation in the gene encoding for LRP6 was found in a family with hyperlipidaemia, type 2 diabetes mellitus and early coronary artery disease [9]. Mutations in various Wnt genes have also been associated with an increased risk for insulin resistance and type 2 diabetes mellitus [101112]. Moreover, circulating levels of the Wnt inhibitor sclerostin were shown to be higher in individuals with prediabetes and type 2 diabetes mellitus compared with normoglycemic individuals [13]. Additionally, the Wnt pathway is involved in adiposity, as mutations that probably impair Wnt signaling are associated with obesity and an increased waist circumference [141516]. In vitro studies have indeed shown that Wnt signaling suppresses adipogenesis and favors precursor cell commitment to other lineages, such as osteoblasts [17].
Altogether, we hypothesized that reduced Wnt signaling contributes to the increased risk to develop an impaired glucose homeostasis and high body fat percentage in South Asians. Therefore, we investigated expression levels of both Wnt and insulin signaling genes in subcutaneous white adipose tissue (WAT) and skeletal muscle as well as plasma sclerostin levels in South Asian and white Caucasian men with prediabetes and overweight or obesity.
Ten Dutch South Asian males with prediabetes and overweight or obesity (body mass index [BMI] 25 to 35 kg/m2, age 40 to 55 years) and 10 age- and BMI-matched Dutch white Caucasian males were enrolled in this study. Participants were recruited via advertisements in local hospitals and universities. Ethnicity was defined as having four grandparents of South Asian or white Caucasian origin. To define South Asian ethnicity, we here adopted the geographical area of South Asia applied by the United Nations, with the exception of Iran (i.e., Afghanistan, Bangladesh, Bhutan, India, The Maldives, Nepal, Pakistan, and Sri Lanka) [18]. Participants underwent a medical screening including their medical history, a physical examination, blood chemistry tests and an oral glucose tolerance test (OGTT) to exclude individuals with undiagnosed type 2 diabetes mellitus according to 2014 American Diabetes Association criteria. Prediabetes was defined as having either a fasting plasma glucose level between 5.6 and 6.9 mmol/L or a plasma glucose level between 7.8 and 11.1 mmol/L at 2 hours after the OGTT [19]. Exclusion criteria included uncontrolled hypertension, hyper- or hypothyroidism, liver or kidney dysfunction, rigorous exercise, smoking and use of β-blockers. Three South Asians were using antihypertensive medication before and during the study, two of whom used an angiotensin-converting enzyme inhibitor and one of whom used an angiotensin II-receptor blocker. The Medical Ethical Committee of the Leiden University Medical Center (LUMC) and Maastricht University Medical Center (MUMC) approved the study protocol (P14-252). The study was undertaken in accordance with the principles of the revised Declaration of Helsinki (2013) and in accordance with the Medical Research Involving Human Subjects Act (WMO). All volunteers provided written informed consent prior to participation.
This study was part of a randomized clinical trial aiming to investigate the effects of L-arginine on brown adipose tissue metabolism (clinical trial registration number: NCT02291458). The study was conducted between November 2014 and October 2015 at the LUMC and MUMC. Participants were instructed to refrain from intense physical exercise for 48 hours prior to the experiment and consumed a standardized evening meal the day before the experiment. After a 10-hour overnight fast, body composition was determined by dual X-ray absorptiometry (Discovery A; Hologic, Marlborough, MA, USA) and a cannula was placed into the right antecubital vein for blood withdrawal. A fasting skeletal muscle biopsy from the musculus vastus lateralis and a subcutaneous WAT biopsy from the umbilical region were obtained under localized anaesthesia. Biopsies were frozen and stored at −80℃ until further analyses.
Skeletal muscle and WAT biopsies were homogenized in 1 mL TriPure RNA Isolation reagent (Roche, Mannheim, Germany) and RNA was extracted according to the manufacturer's instructions. One microgram (1 µg) RNA was reverse-transcribed using a Promega solution kit (Promega, Madison, WI, USA) for cDNA synthesis. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed with a CFX96 PCR device (Bio-Rad, Hercules, CA, USA) using SYBR green (Promega). Primer sequences of genes involved in Wnt and insulin signaling as well as housekeeping genes are listed in Supplementary Table 1. mRNA expression was normalized to ribosomal protein S18 (RPS18) for WAT and LRP10 for skeletal muscle, and expressed as arbitrary units for South Asians compared with White Caucasians using the ΔΔCT method. Due to insufficient RNA yield, data on WAT of one South Asian and one white Caucasian participant were excluded, as well as data on skeletal muscle of one white Caucasian participant.
Plasma sclerostin was measured with an electrochemiluminescence assay (MSD 96-Well MULTI-ARRAY Human Sclerostin Assay; Meso Scale Diagnostics, Rockville, MD, USA), as described previously [20]. Plasma glucose, total cholesterol and triglycerides were measured with an automated spectrophotometer (ABX Pentra 400 autoanalyzer; HORIBA, Kyoto, Japan) with enzymatic colorimetric kits. Plasma insulin was determined with commercially available radioimmunoassay kits (human insulin-specific radioimmunoassay, MilliporeSigma, Burlington, MA, USA). Plasma glycosylated hemoglobin (HbA1c) was measured with ion exchange chromatography (Tosoh G8 HPLC analyser; Sysmex, Kobe, Japan).
Statistical analysis were performed with PASW Statistics version 23.0 for Windows (IBM, Armonk, NY, USA). Baseline characteristics, plasma concentrations, and gene expression levels were compared between South Asians and white Caucasians with two-sided independent sample t-tests. Linear regression analysis was used to perform correlations between Wnt and metabolic parameters. In case of interaction between ethnicity and the correlated parameters, linear regression analysis was performed for South Asians and white Caucasians separately by including ethnicity as a covariate. No adjustments (for e.g., BMI or age) were made, as participants were matched for these parameters. Data are presented as mean±standard error of the mean, unless states otherwise. A P<0.05 was considered statistically significant.
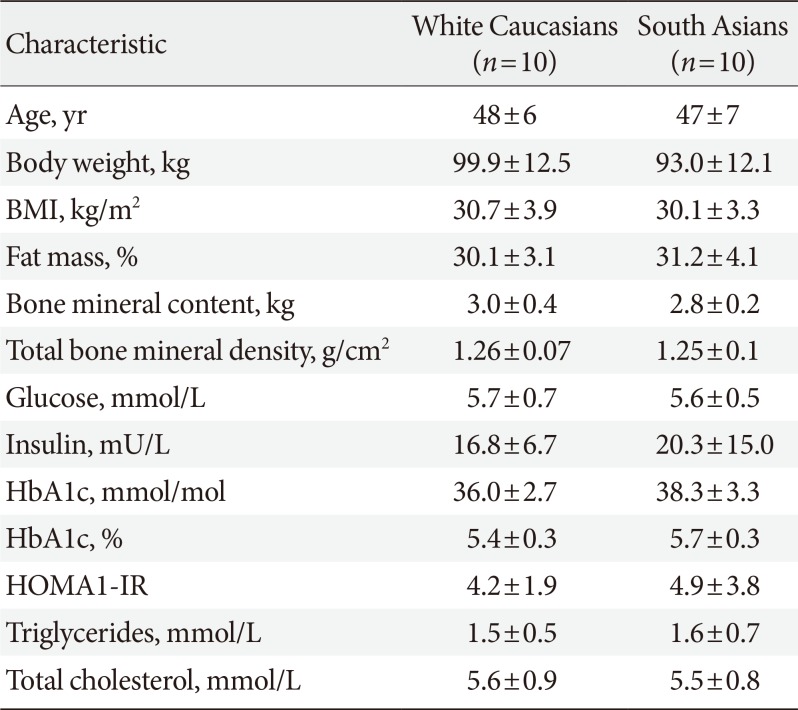
Participant characteristics are summarized in Table 1. In this study, all South Asian subjects were Surinamese-Hindustani with grandparents originating from the Indian subcontinent. All white Caucasians were native Dutch. South Asians and white Caucasians were comparable with respect to age and BMI, as they were matched for these criteria. Plasma glucose, insulin, HbA1c and lipid levels as well as body fat percentage did not statistically significantly differ between South Asians and white Caucasians in this study.
Firstly, we determined plasma sclerostin, a circulating inhibitor of the Wnt signaling pathway (Fig. 1). Sclerostin levels were markedly higher in South Asians compared with white Caucasians (81±8 pg/mL vs. 49±6 pg/mL, P<0.01, respectively). This was not accompanied by a difference in bone mass between South Asians and white Caucasians, as indicated by a similar total bone mineral density (1.25±0.03 g/cm2 vs. 1.26±0.02 g/cm2, P=0.75, respectively). Plasma sclerostin levels negatively correlated with plasma glucose (R2=−0.20, P<0.05) (Supplementary Fig. 1A). We did not observe correlations between plasma sclerostin levels and plasma insulin (Supplementary Fig. 1B), HbA1c (Supplementary Fig. 1C), homeostasis model assessment 1 of insulin resistance (HOMA1-IR) (Supplementary Fig. 1D), or fat mass (data not shown).
To study whether Wnt signaling is altered in metabolic tissues, we investigated expression of Wnt family genes in WAT and skeletal muscle. In addition, we investigated insulin signaling gene expression in both tissues.
In WAT, expression of Frizzled class receptor 1 (FZD1) (−34%, P<0.05) as well as LRP5 (−44%, P<0.05) and LRP6 (−30%, P< 0.05) were lower in South Asians compared with white Caucasians (Fig. 2A). Furthermore, gene expression of the intracellular signal transducer glycogen synthase kinase 3 beta (GSK3B) (−33%, P<0.05) and the transcription factor 7 like 2 (TCF7L2) (−45%, P<0.05) were lower in South Asians (Fig. 2A). Multiple genes involved in insulin signaling (insulin receptor [INSR] −31%, P<0.05; TBC1 domain family member 4 [TBC1D4] −29%, P<0.05; solute carrier family 2 member 4 [SLC2A4] −38%, P<0.05; and glycogen synthase 1 [GYS1] −46%, P<0.05) were also expressed to a lower extent in WAT of South Asians compared with white Caucasians (Fig. 2B). In skeletal muscle, besides a marked lowering of WNT10B expression (−73%, P<0.001), genes involved in Wnt signaling did not differ between South Asians and white Caucasians (Fig. 2C). Genes involved in insulin signaling were also not differentially expressed in skeletal muscle between South Asians and white Caucasians (Fig. 2D).
To investigate whether the lower Wnt signaling gene expression in WAT of South Asians could be related to impaired insulin signaling, we performed correlation analysis between expression of Wnt genes and insulin signaling genes.
We observed a strong positive correlation between expression of multiple Wnt family genes and key insulin signaling genes in WAT. Both LRP5 and LRP6 expression strongly correlated with INSR (LRP5
R2=0.77, P<0.0001; LRP6
R2=0.47, P<0.001) (Fig. 3A and B), TBC1D4 (LRP5
R2=0.67, P<0.0001; LRP6
R2=0.61, P<0.001) (Fig. 3C and D), SLC2A4 (LRP5 R2=0.59, P<0.001; LRP6
R2=0.62, P<0.0001) (Fig. 3E and F) and GYS1 (LRP5
R2=0.85, P<0.0001; LRP6
R2=0.74, P<0.0001) (Fig. 3G and H) expression. In addition, we observed positive correlations between expression of FZD1, frizzled class receptor 8 (FZD8), dishevelled segment polarity protein 2 (DVL2), TCF7L2, AXIN2, and GSK3B and insulin signaling genes (data not shown). For skeletal muscle, we also observed positive correlations between expression of LRP6 and TBC1D4 (R2=0.39, P<0.01) (Supplementary Fig. 2D), SLC2A4 (R2=0.43, P<0.01) (Supplementary Fig. 2F), and GYS1 (R2=0.31, P<0.05) (Supplementary Fig. 2H). Additionally, expression of LRP5 positively correlated with TBC1D4 (R2=0.68, P<0.01) (Supplementary Fig. 2C) and SLC2A4 (R2=0.68, P<0.01) (Supplementary Fig. 2E) in skeletal muscle only in South Asians. Furthermore, expression of DVL2, catenin beta 1 (CTNNB1), TCF7L2, AXIN2, and GSK3B also positively correlated with insulin signaling genes in skeletal muscle (data not shown). We did not observe overt correlations between LRP5 or LRP6 and INSR (Supplementary Fig. 2A and B, respectively), and LRP6 and GYS1 (Supplementary Fig. 2G).
Impaired Wnt signaling is associated with obesity and type 2 diabetes mellitus. As South Asians generally have a high body fat percentage and are at risk for the development of type 2 diabetes mellitus, we hypothesized that Wnt signaling is reduced in South Asian men compared with white Caucasian men. We showed that plasma sclerostin was higher in South Asians without evident effect on its primary target, bone. Additionally, expression of various Wnt family genes in WAT was lower in South Asians than in white Caucasians and this was accompanied by a lower expression of insulin signaling genes. Moreover, there was a strong positive correlation between expression of Wnt family genes and insulin signaling genes in WAT. These observations suggest that Wnt and insulin signaling in WAT are associated and that lower Wnt signaling might, at least in part, affect insulin signaling in South Asians.
Sclerostin is a glycoprotein produced predominantly by mature osteocytes within the mineralized bone matrix, that negatively regulates bone formation via inhibiting canonical Wnt signaling [2122]. We observed higher sclerostin levels in South Asians compared with white Caucasians. Whether these higher sclerostin levels are due to increased production by osteocytes or decreased renal or hepatic elimination, is unknown. Of note, as sclerostin is suggested to be involved in arterial stiffness and vascular calcifications [2324], we critically looked at the plasma sclerostin values of the three South Asian participants with well-controlled hypertension, but these were well within the range of the study population. Interestingly, previous studies showed that circulating sclerostin levels are higher in individuals with prediabetes and diabetes compared with normoglycemic individuals, and that sclerostin levels positively correlate with HbA1c and HOMA-IR [132526272829], albeit we could not reproduce this in our study. We observed a negative correlation between plasma sclerostin and glucose levels, which is against our hypothesis that sclerostin negatively affects glucose homeostasis. However, this fairly weak correlation does not necessarily reflect the effect of altered insulin signaling by sclerostin-induced Wnt inhibition. Moreover, our sample size is limited and previous studies show inconsistent results in different study populations [1327282930]. The mechanism by which sclerostin may affect insulin signaling and glucose homeostasis remains to be established, but likely involves Wnt signaling.
To further investigate Wnt signaling in metabolic tissues, we obtained WAT and skeletal muscle biopsies. Firstly, we consistently observed lower expression of genes involved in Wnt signaling (FZD1, LRP5, LRP6, GSKS3B, and TCF7L2) in WAT of South Asians compared with white Caucasians. Notably, inhibition of canonical Wnt signaling by sclerostin favors differentiation of adipocytes over osteoblastogenesis [3132]. In line with this, in vivo evidence showed that mice with overexpression of sclerostin indeed have reduced WAT expression of genes involved in Wnt signaling and increased expression of genes involved in adipocyte differentiation, together with adipocyte hypertrophy, fat mass accumulation and an impaired glucose homeostasis, whereas the opposite was observed for Sost−/− mice and mice administered a sclerostin-neutralizing antibody [31]. Based on these observations, we speculate that impaired Wnt signaling contributes to the central adiposity that is commonly observed in South Asians. Whether these alterations in the Wnt pathway are congenital or possibly induced by increased sclerostin levels later in life, remains to be explored. Interestingly, Karczewska-Kupczewska et al. [33] also previously showed in an euglycemic clamp study that expression of various Wnt signaling genes in WAT was lower in nondiabetic individuals with low versus high insulin sensitivity. Indeed, in our study expression of key insulin genes (INSR, TBC1D4, SLC2A4, and GYS1) in WAT was also lower in South Asians compared with white Caucasians, suggesting that Wnt and insulin signaling in WAT are associated and that lower Wnt signaling might partially reduce insulin signaling in South Asians.
Aside from a markedly lower WNT10B expression, we did not observe overt differences in skeletal muscle with respect to Wnt and insulin signaling gene expression between South Asians and white Caucasians. It is not unlikely that the canonical Wnt pathway is more involved in adipogenic insulin signaling, as adipocytes and osteoblasts share a direct common cellular progenitor and the involvement of Wnt signaling in adipogenesis is well established [34]. Possibly, Wnt signaling in skeletal muscle is influenced more by other Wnt ligands than sclerostin. Alternatively, Wnt signaling in skeletal muscle might be more regulated by other pathways, including the non-canonical pathway. However, Wnt signaling is likely to affect insulin signaling and sensitivity in skeletal muscle to some extent, as human carriers of an LRP6 loss-of-function mutation have impaired skeletal muscle insulin sensitivity and insulin signaling [11]. In addition, Kim et al. [31] showed that Sost−/− mice had increased skeletal muscle insulin sensitivity compared with control mice, despite unaltered Wnt signaling gene expression in this tissue. In concordance, serum sclerostin levels positively correlated with skeletal muscle and adipose tissue insulin resistance in a hyperinsulinemic-euglycemic clamp study in human subjects with either normoglycemia or prediabetes [13]. Alternative mechanisms that may contribute to peripheral insulin resistance in South Asians are a pro-inflammatory status, including downregulation of genes involved in anti-inflammatory type 1 interferon signaling in WAT and skeletal muscle [35], lower adiponectin levels [3637], as well as a lower skeletal muscle oxidative capacity [38] and cardiorespiratory fitness compared with white Caucasians [39].
Although we cannot draw conclusions regarding causality in the current study set-up, we observed strong correlations between expression of various Wnt family genes, including Wnt coreceptors LRP5 and LRP6, and insulin signaling gene expression in WAT, and to a lesser extent in skeletal muscle. This is in line with evidence showing that Wnt and insulin signaling are intertwined in WAT, as Palsgaard et al. [40] have shown that knocking down LRP5 in murine preadipocytes resulted in reduced phosphorylation of insulin signaling proteins. Whether this is a result of direct interaction between LRP5 and the insulin receptor or due to a mutual downstream docking protein is yet to be unraveled. However, when knocking down LRP5 in human stromovascular cells, Loh et al. [16] did not observe differences in insulin receptor expression or insulin signaling pathway activity.
To the best of our knowledge, this is the first study investigating Wnt signaling and its link with glucose homeostasis in South Asians. A strong point of this study is our ability to investigate Wnt signaling on a tissue-specific metabolic level. Unfortunately, no waist and hip circumference measurements were obtained; therefore, we cannot explore Wnt signaling in relation to body fat distribution in this cohort. The fact that we did not observe correlations between plasma sclerostin levels and HbA1c and HOMA1-IR may be due to our relatively small sample size, as such correlations have repeatedly been shown in larger cohorts. Although we did not observe evident differences in socioeconomical factors between South Asians and white Caucasians, we cannot exclude that socioeconomical status contributed to metabolic differences between ethnicities in this study. It is important to note that the results in these Surinamese-Hindustani cannot be directly extrapolated to other Asian subgroups, as metabolic characteristics differ between Asian populations [41].
To conclude, we show that circulating sclerostin levels are higher and expression of Wnt signaling genes is lower in WAT of South Asian men with prediabetes and overweight or obesity compared with white Caucasian men. Therefore, we speculate that Wnt signaling in adipose tissue might be reduced in South Asians, which could contribute to their susceptibility to develop a disadvantageous metabolic phenotype, including insulin resistance. Future research investigating the interaction between Wnt and insulin signaling is warranted to reveal whether Wnt targeting therapy, such as sclerostin antibodies, can improve insulin sensitivity in South Asians.
ACKNOWLEDGMENTS
Wouter D. van Marken Lichtenbelt receives funding from the EU FP7 project DIABAT (HEALTH-F2-2011-278373) and the Netherlands Organization for Scientific Research (TOP 91209037). Patrick C.N. Rensen is Established Investigator of the Dutch Heart Foundation (2009T038). Mariëtte R. Boon is supported by the Netherlands Organisation for Scientific Research (Rubicon 825.13.021) and the Dutch Diabetes Research Foundation (junior postdoc fellowship; 2015.81.1808).
We thank Hetty C.M. Sips and Trea C.M. Streefland of the Department of Medicine, Division of Endocrinology, LUMC, for their excellent technical assistance.
Notes
AUTHOR CONTRIBUTIONS:
Conception or design: L.G.M.J., A.D.D., S.K., I.M.J., W.D.M.L., P.C.N.R., N.M.A.D., M.R.B.
Acquisition, analysis, or interpretation of data: L.G.M.J., A.D.D., M.J.W.H., S.K., K.J.N., H.R., P.C.N.R., N.M.A.D., M.R.B.
Drafting the work or revising: L.G.M.J., A.D.D., M.J.W.H., S.K., K.J.N., H.R., I.M.J., W.D.M.L., P.C.N.R., N.M.A.D., M.R.B.
Final approval of the manuscript: L.G.M.J., A.D.D., M.J.W.H., S.K., K.J.N., H.R., I.M.J., W.D.M.L., P.C.N.R., N.M.A.D., M.R.B.
References
1. Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016; 118:1723–1735. PMID: 27230638.

2. Middelkoop BJ, Kesarlal-Sadhoeram SM, Ramsaransing GN, Struben HW. Diabetes mellitus among South Asian inhabitants of the Hague: high prevalence and an age-specific socioeconomic gradient. Int J Epidemiol. 1999; 28:1119–1123. PMID: 10661656.

3. Meeks KA, Freitas-Da-Silva D, Adeyemo A, Beune EJ, Modesti PA, Stronks K, Zafarmand MH, Agyemang C. Disparities in type 2 diabetes prevalence among ethnic minority groups resident in Europe: a systematic review and meta-analysis. Intern Emerg Med. 2016; 11:327–340. PMID: 26370238.

4. Haldar S, Chia SC, Henry CJ. Body composition in Asians and Caucasians: comparative analyses and influences on cardiometabolic outcomes. Adv Food Nutr Res. 2015; 75:97–154. PMID: 26319906.
5. Misra A, Ramchandran A, Jayawardena R, Shrivastava U, Snehalatha C. Diabetes in South Asians. Diabet Med. 2014; 31:1153–1162. PMID: 24975549.

6. McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991; 337:382–386. PMID: 1671422.

7. Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007; 19:659–671. PMID: 17188462.

8. Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000; 407:530–535. PMID: 11029007.

9. Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007; 315:1278–1282. PMID: 17332414.

10. Saarinen A, Saukkonen T, Kivela T, Lahtinen U, Laine C, Somer M, Toiviainen-Salo S, Cole WG, Lehesjoki AE, Makitie O. Low density lipoprotein receptor-related protein 5 (LRP5) mutations and osteoporosis, impaired glucose metabolism and hypercholesterolaemia. Clin Endocrinol (Oxf). 2010; 72:481–488. PMID: 19673927.

11. Singh R, De Aguiar RB, Naik S, Mani S, Ostadsharif K, Wencker D, Sotoudeh M, Malekzadeh R, Sherwin RS, Mani A. LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab. 2013; 17:197–209. PMID: 23395167.

12. Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007; 445:881–885. PMID: 17293876.

13. Daniele G, Winnier D, Mari A, Bruder J, Fourcaudot M, Pengou Z, Tripathy D, Jenkinson C, Folli F. Sclerostin and insulin resistance in prediabetes: evidence of a cross talk between bone and glucose metabolism. Diabetes Care. 2015; 38:1509–1517. PMID: 26084344.

14. Christodoulides C, Scarda A, Granzotto M, Milan G, Dalla Nora E, Keogh J, De Pergola G, Stirling H, Pannacciulli N, Sethi JK, Federspil G, Vidal-Puig A, Farooqi IS, O'Rahilly S, Vettor R. WNT10B mutations in human obesity. Diabetologia. 2006; 49:678–684. PMID: 16477437.

15. Guo YF, Xiong DH, Shen H, Zhao LJ, Xiao P, Guo Y, Wang W, Yang TL, Recker RR, Deng HW. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet. 2006; 43:798–803. PMID: 16723389.

16. Loh NY, Neville MJ, Marinou K, Hardcastle SA, Fielding BA, Duncan EL, McCarthy MI, Tobias JH, Gregson CL, Karpe F, Christodoulides C. LRP5 regulates human body fat distribution by modulating adipose progenitor biology in a dose- and depot-specific fashion. Cell Metab. 2015; 21:262–273. PMID: 25651180.

17. Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab. 2009; 20:16–24. PMID: 19008118.

18. United Nations Statistics Division: Methodology: standard country or area codes for statistical use (M49). cited 2019 Sep 9. Available from: https://unstats.un.org/unsd/methodology/m49/.
19. American Diabetes Association. Standards of medical care in diabetes: 2014. Diabetes Care. 2014; 37 Suppl 1:S14–S80. PMID: 24357209.
20. van Lierop AH, Hamdy NA, Hamersma H, van Bezooijen RL, Power J, Loveridge N, Papapoulos SE. Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J Bone Miner Res. 2011; 26:2804–2811. PMID: 21786318.

21. Weivoda MM, Youssef SJ, Oursler MJ. Sclerostin expression and functions beyond the osteocyte. Bone. 2017; 96:45–50. PMID: 27888056.

22. Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005; 280:19883–19887. PMID: 15778503.

23. Appelman-Dijkstra NM, Papapoulos SE. Clinical advantages and disadvantages of anabolic bone therapies targeting the WNT pathway. Nat Rev Endocrinol. 2018; 14:605–623. PMID: 30181608.

24. Chang YC, Hsu BG, Liou HH, Lee CJ, Wang JH. Serum levels of sclerostin as a potential biomarker in central arterial stiffness among hypertensive patients. BMC Cardiovasc Disord. 2018; 18:214. PMID: 30482161.

25. Garcia-Martin A, Rozas-Moreno P, Reyes-Garcia R, Morales-Santana S, Garcia-Fontana B, Garcia-Salcedo JA, Munoz-Torres M. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012; 97:234–241. PMID: 22031520.
26. Gaudio A, Privitera F, Battaglia K, Torrisi V, Sidoti MH, Pulvirenti I, Canzonieri E, Tringali G, Fiore CE. Sclerostin levels associated with inhibition of the Wnt/β-catenin signaling and reduced bone turnover in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012; 97:3744–3750. PMID: 22855334.

27. Yu OH, Richards B, Berger C, Josse RG, Leslie WD, Goltzman D, Kaiser SM, Kovacs CS, Davison KS. The association between sclerostin and incident type 2 diabetes risk: a cohort study. Clin Endocrinol (Oxf). 2017; 86:520–525. PMID: 28090669.

28. Faienza MF, Ventura A, Delvecchio M, Fusillo A, Piacente L, Aceto G, Colaianni G, Colucci S, Cavallo L, Grano M, Brunetti G. High sclerostin and dickkopf-1 (DKK-1) serum levels in children and adolescents with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2017; 102:1174–1181. PMID: 28388723.

29. Gennari L, Merlotti D, Valenti R, Ceccarelli E, Ruvio M, Pietrini MG, Capodarca C, Franci MB, Campagna MS, Calabro A, Cataldo D, Stolakis K, Dotta F, Nuti R. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2012; 97:1737–1744. PMID: 22399511.

30. Wedrychowicz A, Sztefko K, Starzyk JB. Sclerostin and its significance for children and adolescents with type 1 diabetes mellitus (T1D). Bone. 2019; 120:387–392. PMID: 30120991.

31. Kim SP, Frey JL, Li Z, Kushwaha P, Zoch ML, Tomlinson RE, Da H, Aja S, Noh HL, Kim JK, Hussain MA, Thorek DLJ, Wolfgang MJ, Riddle RC. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A. 2017; 114:E11238–E11247. PMID: 29229807.

32. Ukita M, Yamaguchi T, Ohata N, Tamura M. Sclerostin enhances adipocyte differentiation in 3T3-L1 cells. J Cell Biochem. 2016; 117:1419–1428. PMID: 26553151.

33. Karczewska-Kupczewska M, Stefanowicz M, Matulewicz N, Nikolajuk A, Straczkowski M. Wnt signaling genes in adipose tissue and skeletal muscle of humans with different degrees of insulin sensitivity. J Clin Endocrinol Metab. 2016; 101:3079–3087. PMID: 27218273.
34. Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011; 12:722–734. PMID: 21952300.

35. van Dam AD, Hanssen MJW, van Eenige R, Quinten E, Sips HC, Hulsman CJM, Jazet IM, van Marken Lichtenbelt WD, Ottenhoff THM, Haks MC, Rensen PCN, Boon MR. South Asian men have lower expression of IFN signalling genes in white adipose tissue and skeletal muscle compared with white men. Diabetologia. 2017; 60:2525–2528. PMID: 28887664.

36. Mente A, Razak F, Blankenberg S, Vuksan V, Davis AD, Miller R, Teo K, Gerstein H, Sharma AM, Yusuf S, Anand SS. Study of the Health Assessment And Risk Evaluation in Aboriginal Peoples Investigators. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care. 2010; 33:1629–1634. PMID: 20413520.

37. Munoz A, Abate N, Chandalia M. Adipose tissue collagen and inflammation in nonobese Asian Indian men. J Clin Endocrinol Metab. 2013; 98:E1360–E1363. PMID: 23780376.

38. Boon MR, Hanssen MJW, Brans B, Hulsman CJM, Hoeks J, Nahon KJ, Bakker C, van Klinken JB, Havekes B, Schaart G, Jazet IM, Rensen PCN, van Marken Lichtenbelt WD. Effect of L-arginine on energy metabolism, skeletal muscle and brown adipose tissue in South Asian and Europid prediabetic men: a randomised double-blinded crossover study. Diabetologia. 2019; 62:112–122. PMID: 30377712.

39. Ghouri N, Purves D, McConnachie A, Wilson J, Gill JM, Sattar N. Lower cardiorespiratory fitness contributes to increased insulin resistance and fasting glycaemia in middle-aged South Asian compared with European men living in the UK. Diabetologia. 2013; 56:2238–2249. PMID: 23811809.

40. Palsgaard J, Emanuelli B, Winnay JN, Sumara G, Karsenty G, Kahn CR. Cross-talk between insulin and Wnt signaling in preadipocytes. Role of Wnt co-receptor LDL receptor-related protein-5 (LRP5). J Biol Chem. 2016; 291:16878. PMID: 27496960.

41. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363:157–163. PMID: 14726171.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2019.0031.
Supplementary Table 1
Primer sequences for quantitative reverse transcription polymerase chain reaction on white adipose tissue and skeletal muscle
Supplementary Fig. 1
Correlations between plasma sclerostin levels and (A) fasting plasma glucose, (B) insulin, (C) glycosylated hemoglobin (HbA1c), and (D) homeostasis model assessment 1 of insulin resistance (HOMA1-IR) in South Asian and white Caucasian men. Black circles are South Asians and white circles are white Caucasians. Dotted lines represent 95% confidence interval.
Supplementary Fig. 2
Correlations between expression of Wnt signaling coreceptors low-density lipoprotein receptor-related proteins 5 (LRP5) and 6 (LRP6) and insulin signaling genes (A, B) insulin signaling receptor (INSR), (C, D) TBC1 domain family member 4 (TBC1D4), (E, F) solute carrier family 2 member 4 (SLC2A4), and (G, H) glycogen synthase 1 (GYS1) in skeletal muscle (SM) in South Asian and white Caucasian men. Correlations are shown for both groups combined and per ethnicity in case of interaction. Black circles are South Asians and white circles are white Caucasians. Dotted lines represent 95% confidence interval.
Fig. 1
Plasma sclerostin levels in white Caucasian and South Asian men. Data are expressed as mean. Error bars show standard error of the mean. aP<0.01 South Asians vs. white Caucasians.
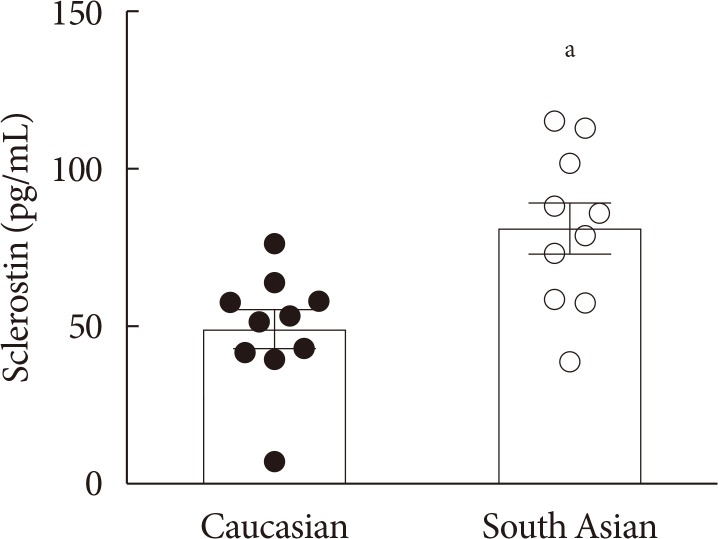
Fig. 2
Gene expression of key players in Wnt and insulin signaling in (A, B) white adipose tissue (WAT) and (C, D) skeletal muscle (SM) in white Caucasian and South Asian men. Data are expressed as mean. Error bars show standard error of the mean. Black bars are South Asians and white bars are white Caucasians. WNT10B, wingless-type MMTV integration site family; FZD, Frizzleds; LRP, low-density lipoprotein receptor-related protein; DVL2, dishevelled segment polarity protein 2; GSK3B, glycogen synthase kinase 3 beta; CTNNB1, catenin beta 1; TCF7L2, transcription factor 7 like 2; INSR, insulin receptor; TBC1D4, TBC1 domain family member 4; SLC2A4, solute carrier family 2 member 4; GYS1, glycogen synthase 1. aP<0.05, bP<0.001, cP<0.1, South Asians vs. white Caucasians.
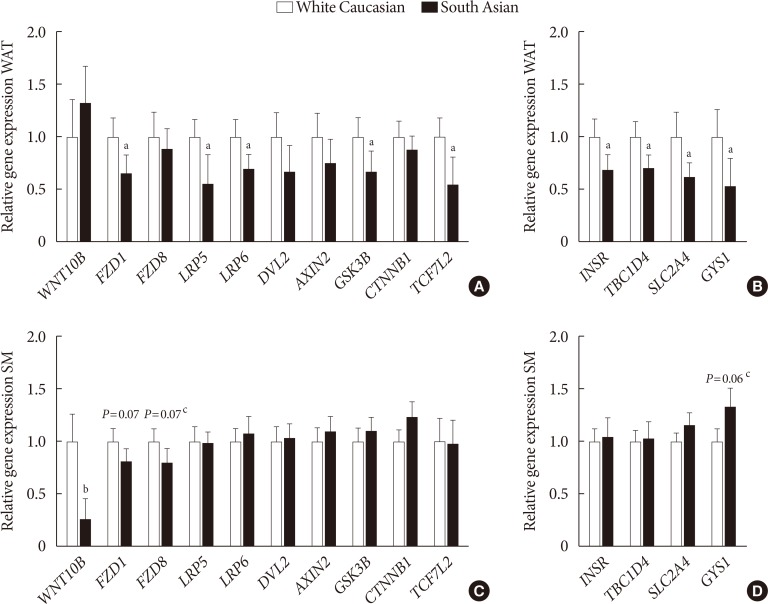
Fig. 3
Correlations between expression of Wnt signaling coreceptors low-density lipoprotein receptor-related proteins 5 (LRP5) and 6 (LRP6) and insulin signaling genes (A, B) insulin signaling receptor (INSR), (C, D) TBC1 domain family member 4 (TBC1D4), (E, F) solute carrier family 2 member 4 (SLC2A4), and (G, H) glycogen synthase 1 (GYS1) in white adipose tissue (WAT) in South Asian and white Caucasian men. Correlations are shown for both groups combined, black circles are South Asians and white circles are white Caucasians. Dotted lines represent 95% confidence interval.
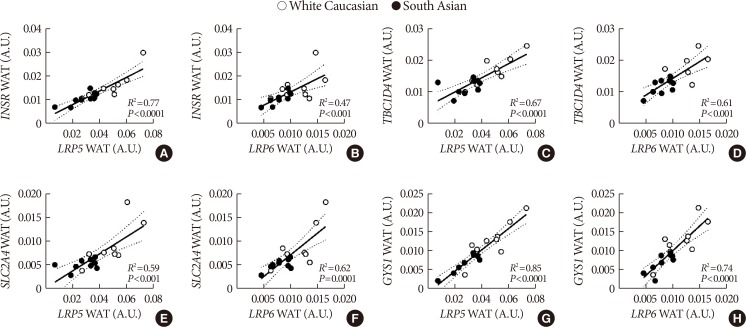
Table 1
Participant characteristics




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download