This article has been
cited by other articles in ScienceCentral.
Abstract
Acute sarcoid myositis is rarely complicated by sarcoidosis, and steroid therapy is considered the standard treatment. We experienced a patient with acute sarcoid myositis who did not respond to aggressive high-dose corticosteroid therapy, but showed a dramatic improvement after the addition of weekly low-dose oral methotrexate (MTX). This intervention allowed the resumption of normal daily activities after 6 months. Our case strongly suggests that MTX should be considered in patients with acute sarcoid myositis that is resistant to corticosteroid therapy.
Go to :

Keywords: Sarcoidosis, Myositis, Corticosteroid, Methotrexate
Sarcoidosis is a multisystem granulomatous disease of uncertain etiology that frequently presents with bilateral hilar lymphadenopathy, pulmonary infiltration, and skin or eye lesions. Steroid therapy is considered the mainstay treatment in most cases [
1]. We present a patient with acute sarcoid myositis who was resistant to high-dose steroid therapy, but showed a dramatic clinical improvement to treatment with the addition of weekly lowdose oral methotrexate (MTX).
CASE
A 49-year-old female presented with rapidly progressive limb muscle weakness that first appeared 2 months previously. She started experiencing difficulties in climbing stairs and arising from a chair, which developed into difficulties of neck control and raising her arms at 1 month after the onset. She denied any other symptom such as myalgia or fever. At presentation, she could neither stand up from a chair by herself nor raise her arms over her head. She lost 10 kilograms of body weight over the 2-month period, and she had a past history of rheumatoid arthritis.
On physical examination, she could not arise from a chair or climb stairs without support, but could walk with a waddling gait. Mild muscle atrophy was observed in the upper arm, shoulder, and thigh. A manual examination of muscle power revealed proximal dominant muscle weakness. The muscle power of the proximal limbs was assessed as Medical Research Council (MRC) grade 3 or 4, while the distal muscles were assessed as MRC grade 4 or 5 (
Table 1). She also showed a profound neck extensor weakness of MRC grade 2, while other cranial muscles were spared. Deep tendon reflexes and sensory function were normal.
Table 1.
Serial changes in muscle power and activities of daily living of the patient
|
At admission |
1 month |
2 months |
3 months |
4 months |
5 months |
6 months |
7 months |
|
Muscle power in modified MRC grade |
|
|
|
|
|
|
|
|
|
|
Neck flexion |
4 |
3 |
3 |
3 |
3 |
3 |
4 |
5 |
|
Neck extension |
2 |
2 |
2 |
3 |
3 |
3 |
4 |
5 |
|
Shoulder elevation |
4 |
3 |
2 |
3 |
4 |
4 |
4 |
4+ |
|
Arm abduction |
4 |
2 |
2 |
2 |
3 |
4- |
4- |
4 |
|
Arm adduction |
4 |
3 |
2 |
3 |
3 |
4- |
4- |
4 |
|
Elbow flexion |
3 |
3 |
3 |
3 |
4- |
4 |
4 |
4 |
|
Elbow extension |
5 |
4 |
4- |
3 |
4 |
4 |
4 |
4 |
|
Wrist flexion |
4+ |
4+ |
4 |
4+ |
5 |
5 |
5 |
5 |
|
Wrist extension |
4+ |
4+ |
4 |
4 |
4+ |
5 |
5 |
5 |
|
Finger grip |
5 |
5 |
4 |
4 |
5 |
5 |
5 |
5 |
|
Finger extension |
5 |
5 |
4 |
4 |
5 |
5 |
5 |
5 |
|
Hip flexion |
3 |
2 |
2 |
2 |
2 |
3 |
4- |
4+ |
|
Hip extension |
4 |
4 |
2 |
2 |
2 |
3 |
4- |
4+ |
|
Knee flexion |
4+ |
4 |
3 |
3 |
4 |
5 |
5 |
5 |
|
Knee extension |
4+ |
4+ |
3 |
4 |
4 |
5 |
5 |
5 |
|
Ankle flexion |
5 |
5 |
4 |
5 |
5 |
5 |
5 |
5 |
|
Ankle extension |
5 |
5 |
4 |
4+ |
5 |
5 |
5 |
5 |
|
MRC Sum-Score |
46 |
40 |
36 |
38 |
44 |
50 |
52 |
52 |
|
Clinical status |
Requires unilateral support |
Requires bilateral support |
Restricted to wheel chair, unable to stand and walk |
Requires bilateral support |
Requires unilateral support |
Walks independently not climbs stairs, runs |
Walks, climbs stairs independently, not runs |
Walks, climbs stairs, runs independently |
|
Treatment |
IV mPDS 1,000 mg/day → oral PDS 50 mg/D |
|
Oral PDS 40 mg/day + weekly oral MTX 15 mg |
|
Oral PDS 35 mg/day + weekly oral MTX 15 mg |
Oral PDS 30 mg/day + weekly oral MTX 15 mg |
Oral PDS 25 mg/day + weekly oral MTX 15 mg |
Oral PDS 20 mg/day + weekly oral MTX 15 mg |

The findings of laboratory tests that included the complete blood count, serum electrolytes, liver, kidney, and thyroid function tests, serum glucose, and C-reactive protein were unremarkable. Antinuclear antibody was positive at a titer of 1:1,280 (cytoplasmic), and rheumatoid factor (31 IU/mL; reference <14 IU/mL) was elevated in serum. The erythrocyte sedimentation rate (37 mm/hr; reference <15 mm/hr) and creatine kinase (CK, 412 IU; reference 5-217 IU) were mildly elevated. A chest roentgenogram and electrocardiogram were unremarkable. The results of nerve conduction studies were within normal limits. Needle electromyography revealed profuse fibrillations and positive sharp waves, with small, short motor-unit potentials in all tested limb muscles, suggesting active myopathic patterns.
Under the clinical impression of a polymyositis, an open muscle biopsy was performed on her left biceps muscle, which revealed numerous noncaseating granulomas in perimysial connective tissue in addition to multifocal lymphohistiocytic infiltration, necrotic and regenerating fibers, and many atrophic muscle fibers, which together strongly suggested a diagnosis of sarcoidosis (
Fig. 1).
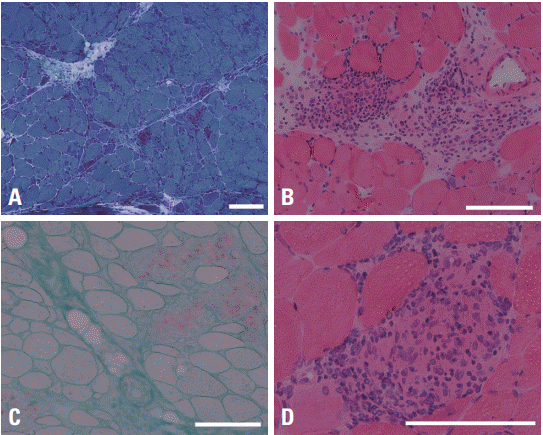 | Fig. 1.Muscle biopsy findings of the patient. Noncaseating granulomas with multinucleated giant cells and infiltrating lymphocytes are observed. In addition, many muscle fibers, especially in the periphery of the muscle fascicles, are atrophic and there are scattered necrotic and regenerating fibers. (A) Modified Gomori-trichrome stain (×40), (B) hematoxylin-eosin stain (×100), (C) acid phosphatase stain (×100), (D) hematoxylin-eosin stain (×200). A white bar indicates 100 μm. 
|
The serum angiotensin-converting enzyme (ACE) level was elevated to 90 U/L (reference 20-70 U/L). Chest computed tomography showed enlargement of bilateral hilar and multiple mediastinal lymph nodes. Whole-body fluorodeoxyglucose positron-emission tomography (FDG-PET) also showed abnormal FDG uptakes in the hilar and mediastinal lymph nodes, but no other extrapulmonary lesion was observed (
Fig. 2). A detailed skin examination revealed subcutaneous macules on the back of the patient, with hyperpigmented macules on the dorsum of the hand and erythematous papules on the forehead. An eye examination did not reveal any significant abnormalities.
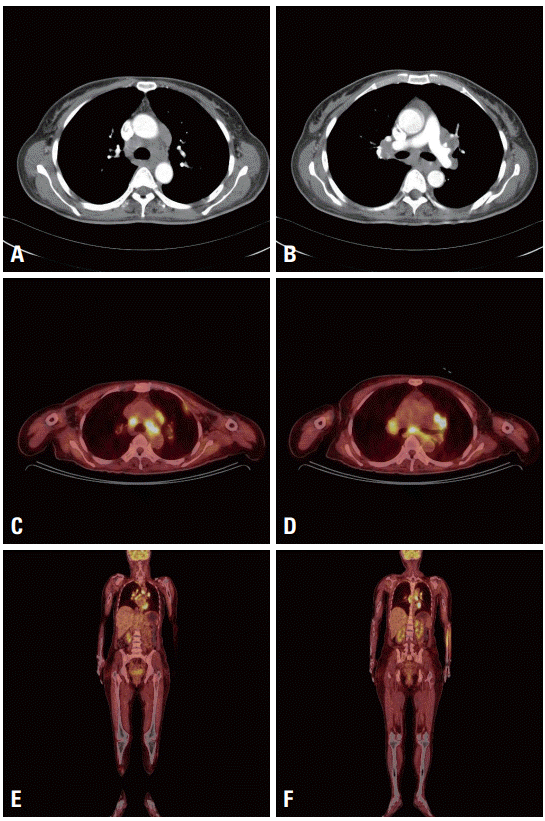 | Fig. 2.A chest computed tomography image shows parenchymal opacifications in the lung and bilateral hilar enlargement with enlarged multiple mediastinal lymph nodes (A, B). Whole-body flourodeoxyglucose positron emission tomography (FDG-PET) shows abnormal FDG uptake in mediastinal lymph nodes and left pleura. In extremities, no abnormal FDG uptake is observed in other tissue including skeletal muscle. An increased FDG uptake in left forearm area is considered as an artifact at injection site (C-F). 
|
After establishing the diagnosis of acute sarcoid myositis associated with pulmonary and skin lesions, high-dose intravenous methylprednisolone therapy (1 g/day for 5 days) was started, followed by oral prednisolone (50 mg/day). Despite this treatment, the patient showed progressive worsening. A second cycle of high-dose corticosteroid therapy was administered 4 weeks later, but produced no benefit. After 7 weeks of corticosteroid therapy the patient became weaker and bedridden, while her serum CK (48 U/L; reference 5-217 IU) and ACE (70 U/L; reference 20-70 U/L) levels had normalized. Her muscle power was assessed as MRC grade 2 in arm abduction and adduction, elbow flexion and extension, trunk flexion, and hip flexion and extension, and MRC grade 3 in neck flexion and extension, and knee flexion and extension (
Table 1).
At 8 weeks after admission we decided to add weekly 15 mg of oral MTX in two divided doses. This intervention produced notable improvements, with the patient improving to walking with assistance after 3 weeks of MTX treatment, walking without assistance after 7 weeks, standing up from the floor by herself after 3 months, and climbing stairs without using hand rails after 5 months (
Table 1). At the last assessment she had normal muscle power in all muscle groups, and had returned to her normal daily living activities on 7.5 mg of daily oral prednisolone and 10 mg of weekly oral MTX.
Go to :

DISCUSSION
Asymptomatic muscle involvement occurs in up to 50% of patients with systemic sarcoidosis, but symptomatic muscle involvement is rare [
1]. Sarcoid myopathy takes three clinical forms: acute sarcoid myositis, palpable nodular type, and chronic myopathy. Acute sarcoid myositis as observed in the present patient is the rarest form, while chronic myopathy is the most common [
2].
Previous studies have shown the usefulness of FDG-PET in identifying active lesions and evaluating therapeutic effects in sarcoid myopathy [
3-
5]. However, the present patient did not show any active muscle lesion on FDG-PET. Considering that FDG-PET was performed before the treatment, and that the patient was in the active stage of disease, the sensitivity of FDG-PET in detecting active sarcoid muscle lesions should be questioned.
Acute sarcoid myositis generally shows favorable response to corticosteroid treatment, while the other forms do not. However, the present patient progressively deteriorated despite receiving vigorous treatment with high-dose corticosteroid, and became bedridden prior to MTX being administered.
In patients with sarcoidosis, various immune suppressants are used as an add-on therapy to a corticosteroid, either due to the steroid-sparing effect or in treatment-resistant cases. Safety issues mean that MTX or azathioprine is usually preferred, and these have been reported as effective in some cases [
6]. In the present case, the addition of weekly low-dose oral MTX led to a dramatic clinical improvement. The patient, who had been bedridden, became able to walk by herself after 7 weeks, and could perform the normal daily living activities after 6 months. Such a dramatic response to low-dose MTX was also recently reported in a Japanese patient with corticosteroid-resistant acute sarcoid myositis [
2]. The present case further supports that MTX should be considered as a second-line treatment option in patients with acute sarcoid myositis when the disease is resistant to corticosteroid therapy.
Go to :

ACKNOWLEDGMENTS
This study was supported by a 2-year (2017-2019) research grant from Pusan National University (Dae-Seong Kim).
Go to :

Notes
Go to :

REFERENCES
1. Silverstein A, Siltzbach LE. Muscle involvement in sarcoidosis. Asymptomatic, myositis, and myopathy. Arch Neurol. 1969; 21:235–241.
2. Fujita H, Ishimatsu Y, Motomura M, Kakugawa T, Sakamoto N, Hayashi T, et al. A case of acute sarcoid myositis treated with weekly low-dose methotrexate. Muscle Nerve. 2011; 44:994–999.

3. Loe MJ, Subramaniam RM, Kalra S, Tiegs RD, Mullan BP, Utz JP. F-18 fluorodeoxyglucose positron emission tomography/computed tomography in the diagnosis of chronic myopathic sarcoidosis. Clin Nucl Med. 2010; 35:22–23.
4. Marie I, Lahaxe L, Vera P, Edet-Samson A. Follow-up of muscular sarcoidosis using fluorodeoxyglucose positron emission tomography. QJM. 2010; 103:1000–1002.

5. Akaike G, Itani M, Shah H, Ahuja J, Yilmaz Gunes B, Assaker R, et al. PET/CT in the diagnosis and workup of sarcoidosis: focus on atypical manifestations. Radiographics. 2018; 38:1536–1549.
6. Baughman RP, Lower EE. A clinical approach to the use of methotrexate for sarcoidosis. Thorax. 1999; 54:742–746.

Go to :







 Citation
Citation Print
Print



 XML Download
XML Download