This article has been corrected. See "Erratum: Correction of Figure. Serotonin Regulates De Novo Lipogenesis in Adipose Tissues through Serotonin Receptor 2A" in Volume 35 on page 672.
Abstract
Background
Obesity is defined as excessive fat mass and is a major cause of many chronic diseases such as diabetes, cardiovascular disease, and cancer. Increasing energy expenditure and regulating adipose tissue metabolism are important targets for the treatment of obesity. Serotonin (5-hydroxytryptophan [5-HT]) is a monoamine metabolite of the essential amino acid tryptophan. Here, we demonstrated that 5-HT in mature adipocytes regulated energy expenditure and lipid metabolism.
Methods
Tryptophan hydroxylase 1 (TPH1) is the rate-limiting enzyme during 5-HT synthesis in non-neural peripheral tissues. We generated adipose tissue-specific Tph1 knockout (Tph1 FKO) mice and adipose tissue-specific serotonin receptor 2A KO (Htr2a FKO) mice and analyzed their phenotypes during high-fat diet (HFD) induced obesity.
Obesity is a chronic disease resulting from an imbalance between energy ingested and energy expended and has been recognized as a major risk factor for type 2 diabetes mellitus, atherosclerosis, metabolic syndrome, cardiovascular diseases, and various types of cancer [1]. In addition, obesity is associated with higher all-cause mortality [2]. Hence, intensive efforts have been taken to uncover the basic mechanisms of obesity and to discover effective therapeutic targets for the treatment of obesity. However, to date, safe and efficacious therapeutics for obesity remain scarce.
Serotonin (5-hydroxytryptophan [5-HT]) is a monoamine metabolite of the essential amino acid tryptophan. Tryptophan is hydroxylated to 5-HT by tryptophan hydroxylase (TPH). TPH represents the rate-limiting step in the pathway, with the enzyme occurring in two isoforms. TPH1 is expressed in non-neural tissues and TPH2 is localized in the neural tissues [3]. In the next step, 5-HT is catalyzed by L-amino acid decarboxylase to produce serotonin. Since serotonin poorly crosses the blood-brain barrier, central, and peripheral serotonin represent separate signaling systems. Peripheral serotonin is produced in enterochromaffin cells in the gastrointestinal tract, pancreatic β-cells, and adipocytes [4]. Serotonin synthesized in the periphery exerts a wide variety of physiological roles by interacting with serotonin receptors in the control of vasoconstriction, intestinal motility, and glucose and lipid metabolism.
Several studies have reported the possible relationships between serotonin and obesity. In humans, the blood serotonin level and Tph1 expression in the duodenum has been positively correlated with body mass index [5]. The genetic analysis reported that Tph1 and 5-HT(2A) receptor, 5-HT(2B) receptor polymorphisms are associated with the development of obesity [6,7]. Furthermore, serotonin synthesis and serotonin levels were reportedly elevated in the liver and adipose tissue of glucocorticoid-induced insulin-resistant rats [8]. These reports suggest that obesity is associated with increased serotonin concentrations in both serum and adipose tissue.
Recent studies have reported that the inhibition of peripheral serotonin results in resistance to obesity and reduced lipid accumulation in peripheral tissues [9–11]. This suggests a link between peripheral serotonin and energy balance. However, the metabolic phenotypes of adipose tissue-specific Tph1 and its receptor knockout mice have not been reported. Therefore, in this study, we generated adipose tissue specific Tph1 knockout (Tph1 FKO) mice and adipose tissue-specific serotonin receptor 2A knockout (Htr2a FKO) mice and analyzed their metabolic phenotypes. We observed that the deletion of Tph1 in adipose tissue resulted in a resistance to high-fat diet (HFD) induced obesity, increased thermogenesis, and reduced lipid accumulation in the adipose tissue. Furthermore, Htr2a FKO mice demonstrated resistance to obesity, with reduced lipid accumulation in the adipose tissue. Our studies contribute to the information regarding serotonin function in adipocyte metabolism and indicate that targeting Tph1 and Htr2a in the adipose tissue could be a potential treatment strategy to manage obesity and related metabolic diseases.
The generation of Tph1-floxed mice, Htr2a-floxed mice, and adiponectin (Adipoq)-Cre mice have previously been reported [11–13]. C57BL/6 J mice were purchased from Charles River Japan (Yokohama, Japan). Tph1 FKO mice were crossed with uncoupled protein 1 (Ucp1)-luciferase transgenic mice. The mice were housed in climate-controlled, specific pathogen-free barrier facilities, under a 12-hour light-dark cycle, with chow and water provided ad libitum. Male mice (aged 8 weeks) were fed either a standard chow diet (SCD; 12% fat calories, Research Diets D10001) or an HFD (60% fat calories, Research Diets D12492). In case of the transgenic mice, we compared the data between KO mice and their wild type (WT) littermates. The experimental protocols for this study were approved by the Institutional Animal Care and Use Committee (LML 15–535) at the Korea Advanced Institute of Science and Technology. These experiments were performed unblinded.
The mice were housed individually in a 12-chamber, open-circuit Oxymax/CLAMS (Columbus Instruments Comprehensive Lab Animal Monitoring system) system and the metabolic rate was measured as previously described [11]. After one day of acclimation, each mouse was assessed for 72 hours in the fed state to assess the metabolic rates. The respiratory exchange ratio (RER=VCO2/VO2) and heat production (HP=3.185×VO2+ 1.232×VCO2) were calculated. Fat mass and lean body mass were measured using Minispec time-domain nuclear magnetic resonance analyzer (Bruker Optics, Billerica, MA, USA).
To perform the glucose tolerance test, the mice were fasted overnight. Then, 2 g/kg D-glucose in phosphate-buffered saline (PBS) was intraperitoneally injected into the mice. For the insulin tolerance test (ITT), 0.75 U/kg human insulin (Humulin R, Lilly, Indianapolis, IN, USA) was intraperitoneally injected into 6 hours fasted mice. Blood samples were collected from the tail vein and glucose concentrations were measured using a Gluco DR plus glucometer (Allmedicus, Anyang, Korea) as previously described [10].
Total RNA extractions from the mice adipose tissues were performed using TRIzol as previously described [14]. After TURBO DNase (Invitrogen, Waltham, MA, USA) treatment, 2 μg of total RNA was used to generate complementary DNA with Superscript III reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. Quantitative real-time polymerase chain reaction (RT-PCR) was performed with Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) and a ViiA 7 Real-time PCR system (Applied Biosystems). Gene expressions were analyzed using the delta-delta Ct method as previously described [11]. The primer sequences are presented in Table 1.
Murine 3T3-L1 cells (American Type Culture Collection) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum and 100 μg/mL penicillin/streptomycin in a humidified atmosphere of 5% CO2 at 37°C. Cells were differentiated and analyzed as previously described [10]. Immunostaining was performed on differentiated day 8.
Inguinal, epididymal, and interscapular adipose tissues were harvested, fixed in 4% (w/v) paraformaldehyde in PBS and embedded in paraffin. Next, 5-μm-thick tissue sections were deparaffinized, rehydrated, and used for hematoxylin and eosin staining, immunohistochemistry and immunofluorescence [10].
We obtained Ucp1-luciferase mice from the laboratory of Dr. Shingo Kajimura (University of California, San Francisco) [15]. Briefly, 150 mg/kg of D-Luciferin potassium salt (GoldBio, St. Louis, MO, USA) was injected into WT and Tph1 FKO with Ucp1-luciferase fed SCD or HFD. Fifteen minutes post-injection, we detected luciferase activity using IVIS Lumina S5, using the Living Image software (PerkinElmer, Waltham, MA, USA) to setup (autoexposure), analyze, and organize data.
The correlations of BXD white adipose tissue (WAT; subcutaneous fat) Tph1 mRNA and phenotypes were analyzed by using database (http://www.genenetwork.org/). The comparison of Tph1 mRNA levels and total 2000 phenotypes correlated with Tph1 in the WAT expressed by the volcano plot. The X axis represents Spearman’s Rho, with positive values representing positive correlations and negative values representing negative correlations, and the y-axis representing inversed P values. The gray horizontal solid line on the y-axis are statistically significant with a P value of less than 0.5, while the vertical solid line on the x-axis has a blue or red color with only Rho values greater than 0.5 (right) or less than −0.5 (left). Expressed in color can be viewed as having a positive or negative correlation. Marked in red is the metabolic phenotype. The result was obtained without bias using the whole without excluding one value from the entire BXD data.
All values are expressed as the mean±standard error of the mean. Statistical significance was determined by Student’s t test or two-way analysis of variance (ANOVA) with Bonferroni’s post hoc test. Two-way ANOVA was performed in body weight (Figs. 1B, 4B), glucose tolerance test (Fig. 1F, Supplemental Fig. S3B), ITT (Supplemental Figs. S2A, S3C), and energy expenditure assays (Fig. 2A, Supplemental Figs. S1E, S2B). P<0.05 was considered statistically significant.
To investigate whether serotonin is associated with body weight and composition, we analyzed RNA expression in WAT of the BXD family from GeneNetwork. Tph1 mRNA expression has shown a positive correlation with fat mass and glucose levels and a negative correlation with lean mass (Fig. 1A). We hypothesized that if serotonin increase by TPH1 in WAT has a positive correlation with increased fat mass, serotonin depletion in WAT may reduce fat mass and body weight gain. Previously, we generated inducible Tph1 knockout (adipocyte protein [aP2]-CreERT2+/−/Tph1 flox/flox) mice and reported that serotonin depletion at the adult stage prevents HFD induced obesity [10]. In this study, we investigated the role of peripheral serotonin in mature adipocytes in the basal status as well as overnutrition status. For this, we generated adipocyte-specific Tph1 knockout (Adipoq-Cre+/−/Tph1 flox/flox, Tph1 FKO) mice and analyzed the phenotypes of these mice at young and mature adult stages (Supplemental Fig. S1A, B). The Ap2 gene can be expressed in adipocyte progenitors and other tissues [16,17]. Notably, Adipoq gene expression is more specific in mature adipocytes than the Ap2 gene expression [18]. In young adults (8 weeks of age), Tph1 FKO mice did not show significant difference in body weight compared to WT mice (Fig. 1B). However, inn mature adults (20 weeks of age), Tph1 FKO mice demonstrated lower body weight and fat mass compared to the WT group when fed a HFD (Fig. 1B, Supplemental Fig. S1C, D). These results are consistent with the correlation analysis of the BXD reference group (Fig. 1A).
To explain the result, we measured the energy expenditure in these mice groups. As expected, Tph1 FKO mice with SCD feeding showed increased energy expenditure (increase VO2, VCO2, and HP) compared to WT mice fed SCD (Supplemental Fig. S1E). Under HFD conditions, after 12 weeks on HFD, these differences observed between Tph1 FKO and WT mice are greater than those observed under SCD conditions. Tph1 FKO fed the HFD showed reduced weight gain and lower body fat mass compared to WT mice (Fig. 1B–D). Notably, Tph1 FKO mice fed the HFD reported an improvement in glucose tolerance and insulin resistance when compared to WT mice fed the HFD (Fig. 1F, Supplemental Fig. S2A).
Tph1 FKO mice fed the HFD demonstrated increased energy expenditure (Fig. 2A, Supplemental Fig. S2B) compared to WT. To elucidate this phenotype, we generated in vivo reporter system for brown adipose tissue (BAT) activity using Ucp1-luciferase reporter mice [15]. Fig. 2B shows increased luciferase activity in the BAT area of Tph1 FKO and WT mice. Tph1 FKO mice fed the SCD and HFD showed significantly increased luciferase activity compared to WT mice fed the SCD and HFD (Fig. 2C). Then, we analyzed the histologic changes in BAT by aging. At 8 weeks of age, brown adipocytes in Tph1 FKO mice and WT mice appeared identical (Fig. 2D). This is consistent with their body weight at 8 weeks of age (Fig. 1B). As the mice grew older, from 8 to 20 weeks of age, the size of adipose cells and lipid droplets increased. However, Tph1 FKO BAT maintained a similar size of adipose cells at 20 weeks of age, even after 12 weeks of HFD (Fig. 2D).
Previously, we reported that serotonin depletion in the subcutaneous adipose tissue induced beige adipocyte formation in mice fed the HFD [10]. We observed similar changes after depleting serotonin in mature subcutaneous adipose tissue after HFD (Fig. 3A). Interestingly, serotonin depletion also increased beige adipocyte formation in the subcutaneous adipose tissue of mice fed the SCD (Fig. 3B). In visceral adipose tissue, Tph1 FKO mice fed HFD demonstrated a reduced adipose cell size and lipid droplets compared to WT mice fed on the HFD (Fig. 3C, D). Under the SCD conditions, Tph1 FKO mice did not indicate significant differences in the visceral adipose tissue compared to WT mice (Fig. 3C).
Previously, we identified the role of HTR2A in 3T3-L1 adipocytes [10]. In 3T3-L1 cell lines, HTR2A agonist increased lipogenesis gene expression in differentiated 3T3-L1 adipocytes [10]. Using these in vitro data, we suggested that HTR2A may regulate lipogenesis in WAT. To evaluate this hypothesis, we performed both in vitro and in vivo studies. We observed the lipid accumulation process in the in vitro system. We stained differentiated 3T3-L1 adipocytes with lipid (green, BODIPY 493/503) and serotonin (red, 5-HT). Fig. 4A shows the presence of serotonin and lipid droplets in differentiated 3T3-L1 adipocytes. Interestingly, we observed 5-HT and BODIPY copositive adipocytes (white arrowhead, Fig. 4A). The yellow staining implied that serotonin is related to lipid accumulation. Accordingly, the lipogenic gene and Htr2a gene expressions, in 3T3-L1 adipocytes, were increased during differentiation (Supplemental Fig. S3A).
Next, we generated adipocyte-specific Htr2a knockout (Adipoq-Cre+/−/Htr2a flox/flox, Htr2a FKO) mice. Htr2a FKO mice fed the HFD demonstrated a lower body weight gain and improved glucose tolerance compared to WT mice fed the HFD (Fig. 4B, Supplemental Fig. S3B, C). As expected, the adipose cell size and lipid droplets were decreased not only in the visceral adipose tissue but also in subcutaneous and BAT of Htr2a FKO mice (Fig. 4C, D, Supplemental Fig. S3D, E). The gene expression analysis also showed reduced lipogenic gene expression in Htr2a FKO mice (Fig. 4E). These in vivo data strongly suggested that HTR2A in WAT regulates lipogenesis.
Recent studies have reported that serotonin regulates energy metabolism in peripheral tissues such as the adipose tissue and liver [9–11,19]. Regarding the HFD condition, HFD increases serotonin levels in the adipose tissue [10]. The inhibition of serotonin synthesis increased energy expenditure and thermogenesis in BAT of mice fed the HFD [9,10]. In the liver, gut-derived serotonin regulates lipid accumulation [11,19]. These data consistently show a strong association between serotonin and lipid metabolism.
In this study, we investigated the role of serotonin in mature adipocytes under basal and overnutrition conditions. When we depleted serotonin levels genetically, the mice demonstrated resistance to obesity, increased energy expenditure, and elevated BAT activity (Figs. 1B–E, 2A–D). The WAT of HFD-fed mice maintained similar sizes of cell and lipid droplets to adipocytes in SCD-fed mice (Fig. 3C), suggesting that high serotonin leads to obesity-prone adipocytes by increasing lipogenesis and reducing thermogenesis.
In terms of energy metabolism, we observed an increased HP in the mice fed SCD as well as HFD (Fig. 2A, Supplemental Fig. S1E). Under low serotonin conditions, UCP1 activity in BAT and beige adipocyte were increased in the subcutaneous adipose tissue of both SCD- and HFD-fed Tph1 FKO mice (Figs. 2C, 2D, 3A, 3B). This implied that basal serotonin in mature adipocytes suppresses thermogenic activity in BAT and beige adipocyte formation in WAT. Furthermore, this finding expands the clinical importance of peripheral serotonin. If serotonin suppresses energy dissipation even under basal conditions, the anti-obesity effect of serotonin inhibition may be observed irrespective of the serotonin levels in adipose tissue. Intriguingly, Tph1 FKO mice demonstrated an increased food intake during both SCD and HFD feeding (Supplemental Figs. S1E, S2B). However, we failed to elucidate the exact underlying mechanism which could explain this behavioral change. The secondary effects could be attributed to the increased energy expenditure or unknown alterations in the lipid-brain axis due to serotonin deficiency. Nonetheless, Tph1 FKO mice demonstrate resistance to obesity despite the increased food intake.
With regard to lipogenesis in WAT, we focused on HTR2A. Several studies, including our group, have shown that HTR2A has a role in lipogenesis in 3T3-L1 cells [10,20]. In this study, we generated Htr2a FKO mice and observed that serotonin depleted WAT demonstrated reduced adipose cell sizes and lipid droplets (Fig. 4C, D), suggesting that HTR2A mediates the lipogenic changes in WAT. Moreover, these changes were only observed in the WAT of HFD-fed Htr2a FKO mice. The WAT of SCD-fed mice was similar to the WAT of WT mice. This implied that elevated serotonin, not basal serotonin, regulates lipogenesis in WAT under overnutrition conditions.
We reported that HTR3 is the responsible receptor that regulates thermogenesis in BAT [10]. Concerning the formation of beige adipocytes in the subcutaneous adipose tissue, we observed several UCP1 positive adipocytes in the subcutaneous adipose tissue of Tph1 FKO mice (Fig. 3B). This implied that the inhibition of serotonin synthesis induced beige adipocyte formation. However, the exact process of beige adipocyte formation after serotonin depletion remains unclear, necessitating further evaluation of the role of serotonin in the fate of pre-adipocyte, UCP1 positive adipocyte recruitment, and activation. Furthermore, we plan to investigate the responsible receptors regulating this change in the subcutaneous adipose tissue.
In conclusion, adipocyte-derived serotonin regulates lipogenesis and thermogenesis in the adipose tissue. Inhibition of serotonin synthesis in mature adipocytes reduced lipid accumulation in visceral WAT, induced beige formation in subcutaneous WAT and increased thermogenesis in BAT. In addition, serotonin increased lipogenesis through HTR2A signaling in visceral adipose tissue. Therefore, HTR2A inhibition, as well as TPH1 inhibitor, might be an effective treatment strategy for obesity, especially in case of visceral obesity.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1A6A3A04010466 to Chang-Myung Oh, 2019M3A9A8066460 to Sangkyu Park, and 2016M3A9B6902871 to Hail Kim). BXD family mice data were taken from GeneNetwork database (www.genenetwork.org).
REFERENCES
1. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006; 444:875–80.

2. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013; 309:71–82.
3. Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003; 299:76.

4. Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007; 132:397–414.

5. Young RL, Lumsden AL, Martin AM, Schober G, Pezos N, Thazhath SS, et al. Augmented capacity for peripheral serotonin release in human obesity. Int J Obes (Lond). 2018; 42:1880–9.

6. Li P, Tiwari HK, Lin WY, Allison DB, Chung WK, Leibel RL, et al. Genetic association analysis of 30 genes related to obesity in a European American population. Int J Obes (Lond). 2014; 38:724–9.

7. Kwak SH, Park BL, Kim H, German MS, Go MJ, Jung HS, et al. Association of variations in TPH1 and HTR2B with gestational weight gain and measures of obesity. Obesity (Silver Spring). 2012; 20:233–8.

8. Li T, Guo K, Qu W, Han Y, Wang S, Lin M, et al. Important role of 5-hydroxytryptamine in glucocorticoid-induced insulin resistance in liver and intra-abdominal adipose tissue of rats. J Diabetes Investig. 2016; 7:32–41.

9. Crane JD, Palanivel R, Mottillo EP, Bujak AL, Wang H, Ford RJ, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2015; 21:166–72.

10. Oh CM, Namkung J, Go Y, Shong KE, Kim K, Kim H, et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun. 2015; 6:6794.

11. Choi W, Namkung J, Hwang I, Kim H, Lim A, Park HJ, et al. Serotonin signals through a gut-liver axis to regulate hepatic steatosis. Nat Commun. 2018; 9:4824.

12. Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008; 135:825–37.

13. Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011; 13:249–59.

14. Rio DC, Ares M Jr, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc. 2010; 2010:pdb.prot5439.

15. Galmozzi A, Sonne SB, Altshuler-Keylin S, Hasegawa Y, Shinoda K, Luijten IHN, et al. ThermoMouse: an in vivo model to identify modulators of UCP1 expression in brown adipose tissue. Cell Rep. 2014; 9:1584–93.

16. Shan T, Liu W, Kuang S. Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues. FASEB J. 2013; 27:277–87.

17. Lee KY, Russell SJ, Ussar S, Boucher J, Vernochet C, Mori MA, et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013; 62:864–74.

18. Chakrabarti P. Promoting adipose specificity: the adiponectin promoter. Endocrinology. 2010; 151:2408–10.

Fig. 1
Tryptophan hydroxylase 1 (Tph1) knockout (FKO) protects against high-fat diet (HFD)-induced obesity. (A) Volcano plot for mRNA expression of adipose tissue from the BXD strains. (B) Bodyweight curves in wild type (WT) and Tph1 FKO mice fed standard chow diet (SCD) or HFD for 12 weeks. Bodyweight of WT and Tph1 FKO mice were measured weekly from week 4 to week 20; n=5 in each group fed SCD, n=8 in each group fed HFD. (C). Body fat and lean body mass of mice after 12 weeks of HFD feeding. (D, E) Gross appearance and fat mass of visceral, inguinal and brown fat of 20-week-old WT and Tph1 FKO mice (left) fed on HFD. Adipose tissue weight (right); n=7 in each group. (F) Glucose tolerance tests. Blood glucose concentrations were measured at the indicated time points after fasted for 16 hours; n=10 in each group fed HFD. iWAT, inguinal white adipose tissue; eWAT, epidydimal white adipose tissue; BAT, brown adipose tissue. aP<0.05; bP<0.01 indicated significance.
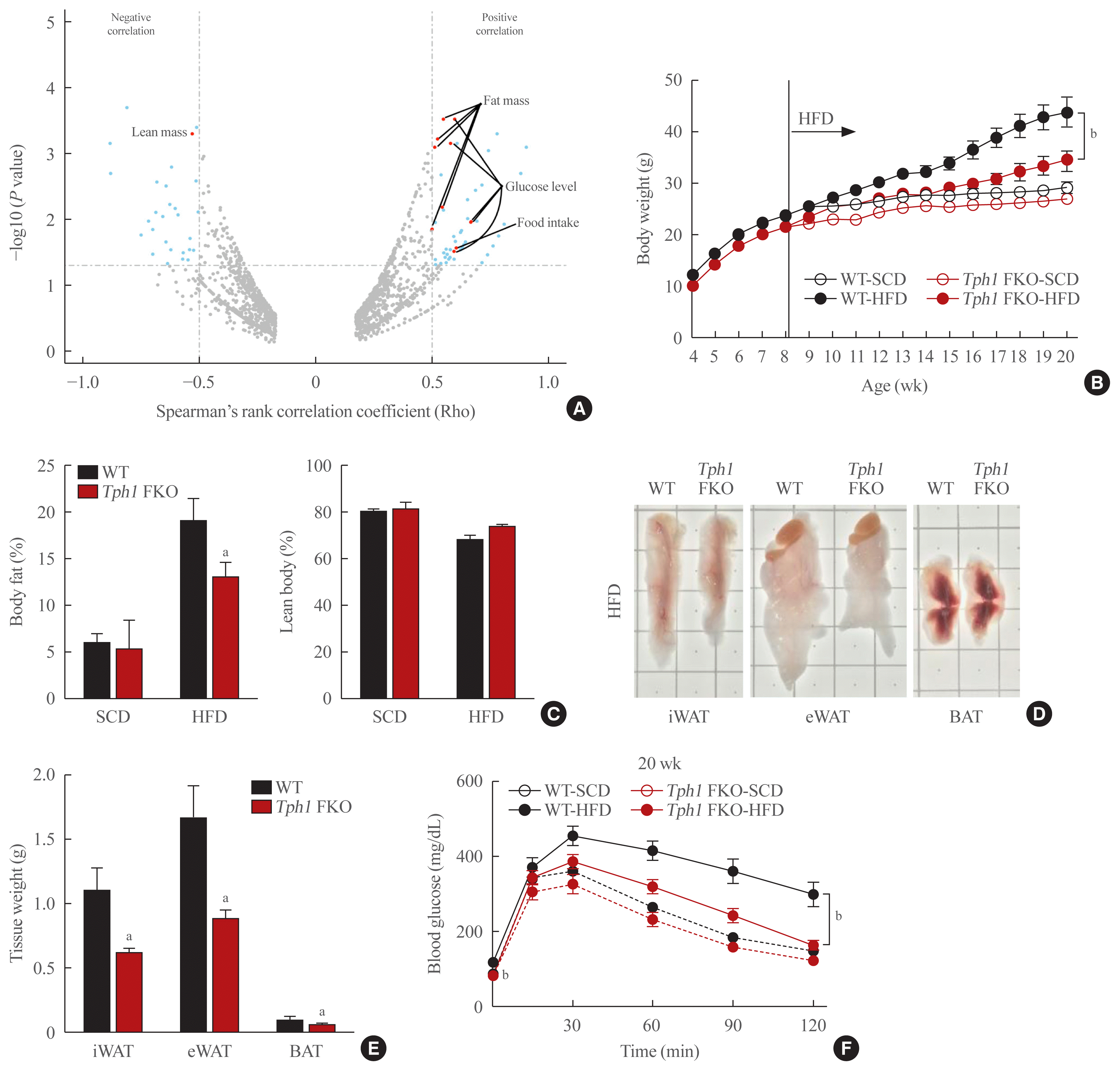
Fig. 2
Tryptophan hydroxylase 1 (Tph1) knockout (FKO) increased energy expenditure. (A) Oxygen (O2) consumption (left), carbon dioxide (CO2) production (middle) and heat production (right) in wild type (WT) and Tph1 FKO mice fed on high-fat diet (HFD) for 12 weeks; n=8 in each group fed HFD. (B, C) In vivo luciferase assay of WT and Tph1 FKO mice. Tph1 FKO mice show increased uncoupled protein 1 (UCP1) activity. (D) Histological analysis of brown adipose tissue (BAT) at the indicated age. Adipose tissue sections were stained with H&E (scale bar, 100 μm). SCD, standard chow diet. aP<0.05; bP<0.01 indicated significance.
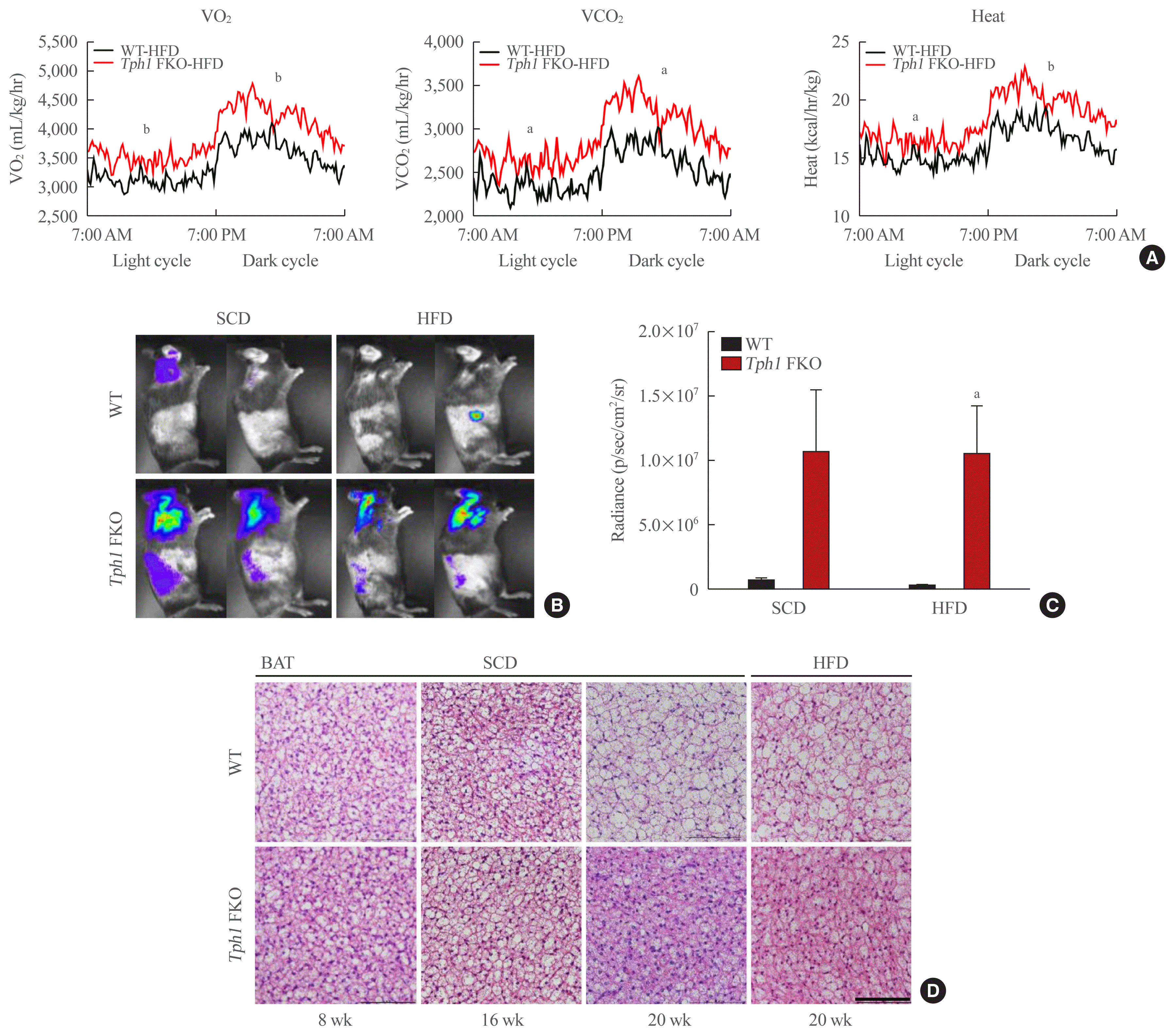
Fig. 3
Representative images of adipose tissues of tryptophan hydroxylase 1 (Tph1) knockout (FKO) mice. (A) Histological analysis of iWAT at the indicated age. Adipose tissue was stained with H&E. (B) Immunostaining of UCP1 in iWAT (scale bar, 100 μm). (C) Histological analysis of eWAT at the indicated age. Adipose tissue sections were stained with H&E (scale bar, 100 μm). (D) The adipocyte size was analyzed using ImageJ software (NIH). iWAT, inguinal white adipose tissue; UCP1, uncoupled protein 1; eWAT, epididymal white adipose tissue; SCD, standard chow diet; HFD, high-fat diet; WT, wild type. aP<0.05 indicated significance.
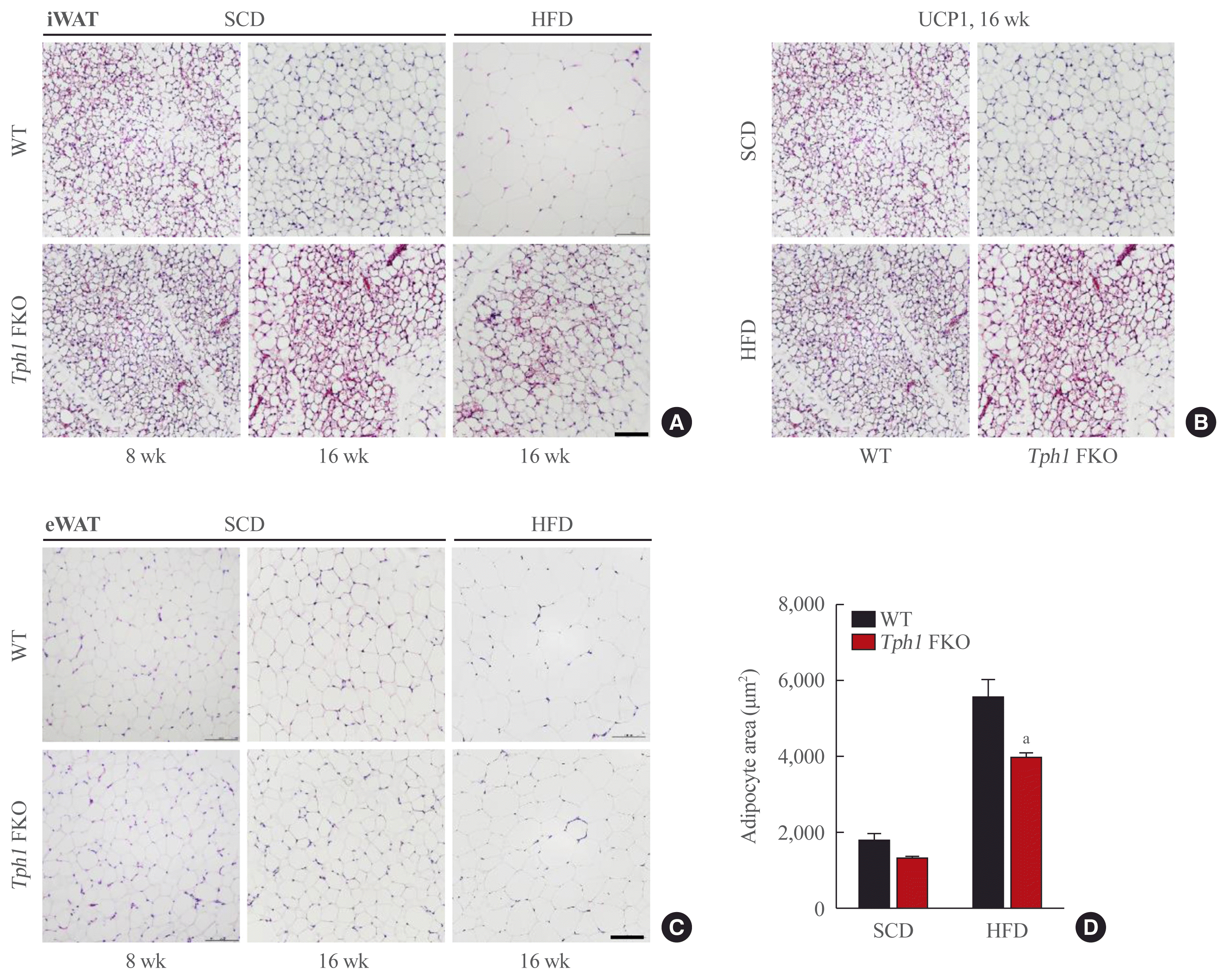
Fig. 4
Inhibition of adipose tissue-specific serotonin receptor 2A (HTR2A) signaling reduces lipid accumulation in white adipose tissue (WAT). (A) Representative images of immunofluorescence staining of differentiated 3T3-L1 adipocytes with BODIPY (green), anti-5-hydroxytryptophan (5-HT) antibody (red), and 4′,6-diamidino-2-phenylindole (DAPI; blue). White arrow indicates BODIPY and 5-HT co-positive cells. (B) Body weight curves in wild type (WT) and Htr2a FKO mice fed standard chow diet (SCD) or high-fat diet (HFD) for 12 weeks. (C) Histological analysis of epididymal white adipose (eWAT) in of WT and Htr2a FKO mice fed SCD or HFD. (D) The adipocyte sizes were analyzed using the ImageJ program. (E) mRNA expression level of fatty acid synthesis (Acaca, Fasn, Scd1), triglyceride synthesis (Dgat1, Dgat2, Gpam, Mogat1), and Plin1. Acaca, acetyl-coA carboxylase alpha; Fasn, fatty acid synthase; Scd1, stearoyl-CoA desaturase 1; Dgat1, diacylglycerol O-acyltransferase 1; Dgat2, diacylglycerol O-acyltransferase 2; Gpam, glycerol-3-phosphate acyltransferase, mitochondrial; Mogat1, monoacylglycerol O-acyltransferase 1; Plin1, perilipin 1. aP<0.05; bP<0.001 indicated significance.
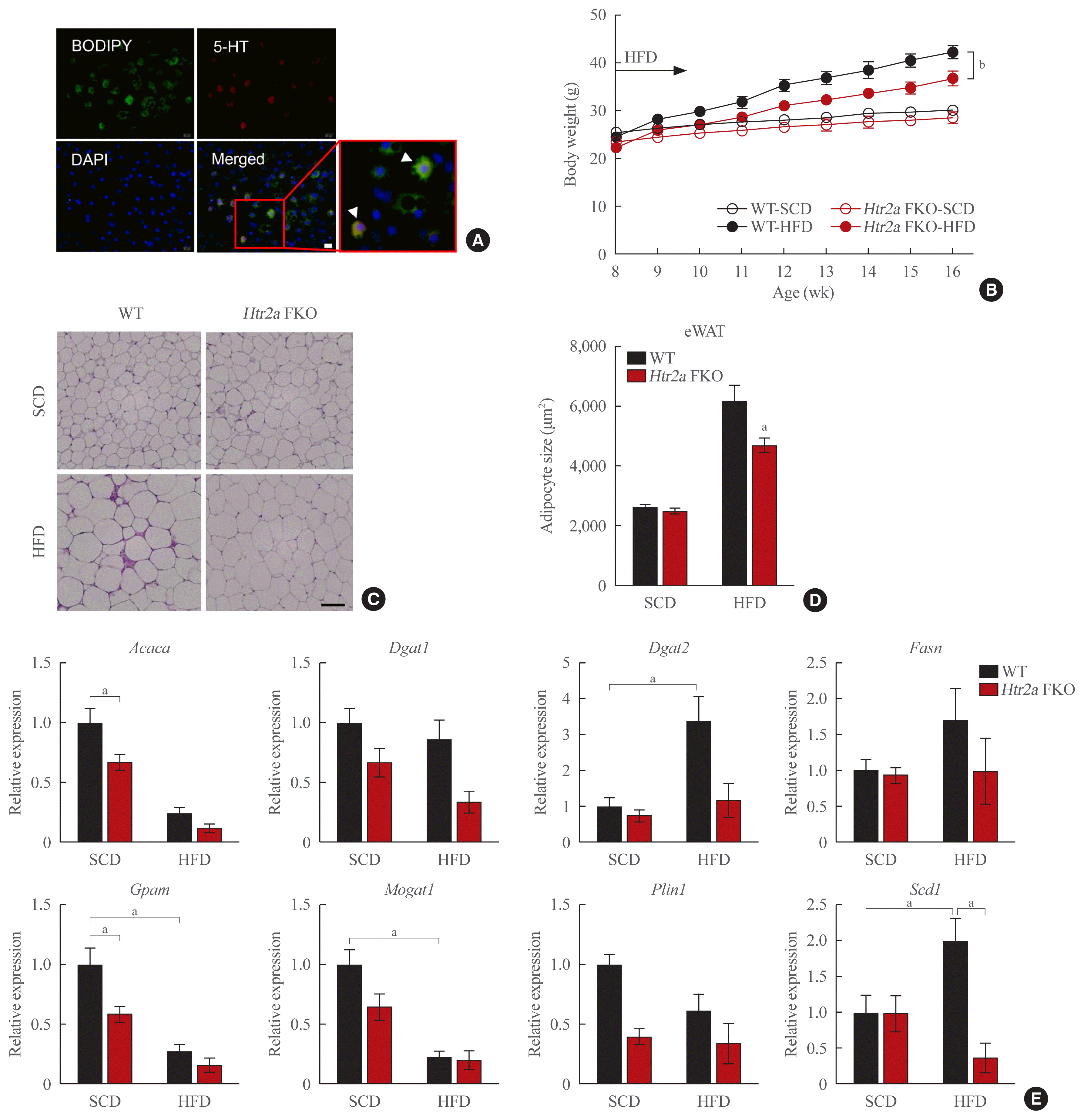
Table 1
List of Primers Used for Real Time Quantitative Polymerase Chain Reaction
Actb, beta-actin; Tph1, tryptophan hydrxylase 1; Ucp1, uncoupled protein 1; Cidea, cell death-inducing DNA fragmentation factor alpha-like effector A; Dio2, iodothyronine deiodinase 2; Pgc1a, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; Prdm16, PR/SET domain 16; Adipoq, adiponectin; Pparg, peroxisome proliferator-activated receptor gamma; Cd36, cluster of differentiation 36; Dgat1, diacylglycerol O-acyltransferase 1; Fasn, fatty acid synthase; Acaca, acetyl-coA carboxylase alpha; Hsl, hormone sensitive lipase; Atgl, adipose triglyceride lipase.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download